Home > A. Molecular pathology > BCL2
BCL2
MIM.151430 18q21.3
Wednesday 29 October 2003
| MIM.151430 | HGNC:990 | WP | PO |
Definition: BCL2 is a proto-oncogene located at 18q21 that promotes B-cell survival via inhibition of apoptosis and confers chemotherapy resistance. BCL2 encodes 25 kDa protein, mainly localized to inner mitochondrial membrane; also endoplasmic reticulum and nuclear envelope.
Pathology
IGH/BCL2 reaerangement by t(14;18)(q32;q21) in follicular B-cell lymphoma (70-80% of cases) (14q32 and 18q21).
- BCL2 deregulation is most commonly associated with the t(14;18), present in approximately 15% of DLBCLs.
- BCL2 protein expression can be detected in approximately 50% of DLBCLs, independent of the t(14;18).
- Increased expression of the BCL2 protein is associated with an inferior outcome in DLBCL, though the t(14;18) alone has no predictive value.
- Because the t(14;18) is the hallmark abnormality in follicular lymphoma and transformed follicular lymphomas resemble de novo DLBCL, the reported frequencies of t(14;18) in DLBCL series may depend on how accurately included tumors and patients were pre-screened.
Diagnostic use
follicular lesions
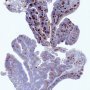
- Distinguish follicular hyperplasia of lymph node (germinal centers are bcl2 negative) from follicular lymphoma (germinal centers are bcl2+);
- bcl2 usually overexpressed in follicular lymphoma due to t(14,18)(q32;q21), which brings bcl2 gene adjacent to active immunoglobulin heavy chain (IgH) gene; however, some follicular lymphomas are bcl2 negative (Am J Surg Pathol 2011;35:1691, Am J Surg Pathol 2009;33:591)
neurons
- Detect immature enteric ganglion cells in pediatric intestinal pseudo-obstruction (Am J Surg Pathol 2005;29:1017)
Diffuse large B-cell lymphoma (DLBCL)
- Diffuse large cell lymphoma: adverse prognostic factor in some studies (Mod Pathol 2005;18:1113, Clin Cancer Res 2011 Sep 20 [Epub ahead of print])
Myelodysplastic syndrome
- increased expression associated with progression
mammary carcinomas
- May have prognostic value in early stage breast cancer (Br J Cancer 2010;103:668)
Expression in normal cells
BCL2 Expression in tumors
hematopoietic tumors
- follicular lymphoma (germinal centers stain);
- acute lymphoblastic leukemia,
- angiotropic / intravascular lymphoma,
- Burkitt lymphoma (20-30%) (occasionally, Indian J Pathol Microbiol 2011;54:290),
- diffuse large B cell lymphoma (variable),
- extranodal NK/T-cell lymphoma, nasal-type: 39% (Am J Clin Pathol 2008;130:343),
- Hodgkin lymphoma (classical): 27% (Hum Pathol 2007;38:103),
- mantle cell lymphoma,
- marginal zone lymphoma (variable),
- persistent polyclonal lymphocytosis,
- plasmablastic lymphoma,
- SLL
adrenal cortical adenoma and carcinoma (Mod Pathol 1998;11:716),
atrioventricular node tumor of heart,
basal cell carcinoma (skin and prostate, Am J Surg Pathol 2007;31:697)
breast tumors
- breast-benign stromal spindle cell tumor,
- breast-columnar cell lesions,
- breast-micropapillary carcinoma,
- breast-phyllodes tumor (stromal cells)
- mammary small cell carcinoma
cervix-mesonephric remnants / rests,
cervix-tuboendometrial metaplasia,
collecting duct carcinoma-kidney,
colorectal adenomas / carcinomas,
large cell neuroendocrine carcinoma (salivary glands),
lymphoepithelioma-like carcinoma,
melanoma (uvea, Arch Pathol Lab Med 1996;120:497),
pheochromocytoma (variable)
spindle cell epithelioma of vagina (Arch Pathol Lab Med 2001;125:547),
squamous cell carcinoma (uterus),
squamous papilloma (conjunctiva, Ann NY Acad Sci 2004;1030:419)
soft tissue tumors
- synovial sarcoma (Hum Pathol 1996;27:1060)
- solitary fibrous tumor
- dermatofibrosarcoma protuberans
- giant cell angiofibroma,
- "hemangiopericytoma"
- sclerosing epithelioid fibrosarcoma,
- myofibroblastoma
- low-grade fibromyxoid sarcoma of soft tissue
thyroid tumors
- thyroid gland-CASTLE
BCOR-CCNB3 Ewing-like sarcoma (90%) (24805859)
Negative lesions
anaplastic lymphoma
apocrine benign / malignant lesions
benign fibrous histiocytoma
germinal center in nodular lymphoid hyperplasia (marginal zone cells in hyperplastic areas are bcl2+, germinal centers are bcl2-)
lymphoplasmayctic lymphoma
Functions
BCL2 prevents cells from undergoing apoptosis.
BCL2 overexpression increase lifespan of B cells; may maintain memory B cells, plasma cells and neurons by prolonging life span without cell division
BCL2 may participate in ion channel formation and alteration of membrane permeability, necessary for initiation of apoptosis.
Non-phosphorylated bcl2 inhibits apoptosis, and Bax homodimers normally cause apoptosis; Bax can bind to and inhibit non-phosphorylated bcl2, promoting apoptosis
Epstein-Barr virus latent membrane protein-1 (LMP-1) interacts with bcl2 promoter, leading to prolonged lifespan for B cells
Surprisingly, bcl2 overexpression in osteoblasts causes osteocyte apoptosis (PLoS One 2011;6:e27487)
Bcl2 family
The BCL2 family includes both antiapoptotic and proapoptotic members that form heterodimers and homodimers.
Following death signals, proapoptotic homodimers alter mitochondrial membrane potential, trigger cytochrome c release, and caspase-mediated apoptosis.
Increased abundance of antiapoptotic BCL2 proteins favors the formation of antiapoptotic/proapoptotic heterodimers rather than proapoptotic/proapoptotic homodimers, limiting the effects of death signals at the mitochondrial membrane.
Either the relative excess of antiapoptotic BCL2 family members or the deficiency of proapoptotic isoforms may confer a survival advantage and contribute to lymphomagenesis.
BCL2 expression is normally down-regulated in the GC where apoptosis plays a critical role in negative B-cell selection.
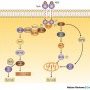
Anti-apoptosis
In a viable cell, the proapoptotic Bcl-2 family members Bax, Bak, and BH3-only proteins are antagonized by antiapoptotic members such as Bcl-2.
In response to an apoptotic stimulus, BH3-only members are activated by transcriptional upregulation (Bax, Noxa, Puma), subcellular relocalization (Bim, Bmf), dephosphorylation (Bad), or proteolysis (Bid).
Activated BH3-only proteins prevent antiapoptotic Bcl-2 members from inhibiting proapoptotic members. In addition, they might directly induce a conformational change of Bax and Bak which subsequently oligomerize and insert into the mitochondrial membrane where they form pores either by themselves or by associating with the permeability transition pore complex.
In consequence, proapoptotic factors are released from the inner mitochondrial membrane into the cytosol, such as cytochrome c which contributes to the formation of the apoptosome and the subsequent activation of the caspase cascade.
Pathology
follicular lymphoma
- Impaired apoptosis is both a critical step in the development of cancer and a major impediment to effective therapy.
- Bcl-2, the oncoprotein activated via the t(14;18) chromosome translocation associated with human follicular lymphoma, inhibits cells from undergoing apoptosis in response to a variety of intracellular damage signals, including those evoked by radiation and chemotherapeutic agents.
BCL2 family
A score or so Bcl-2 relatives have now been identified in mammalian cells and, while the closest homologs (Bcl-xL, Bcl-w, Mcl-1 and A1) are also anti-apoptotic, others are instead pro-apoptotic.
Bax and Bak are very similar to Bcl-2 in sequence, particularly in three conserved Bcl-2 Homology regions (BH1, BH2 and BH3) and, judging by Bax, also in structure.
In contrast, the ’BH3-only proteins’ are largely unrelated, apart from the signature BH3 domain that is essential for their killing function. Collectively, the Bcl-2 family functions as a ’life/death switch’ that arbitrates whether or not a cell should activate the caspase-driven proteolytic cascade responsible for cellular demolition.
Damage signals activate ’BH3-only’ proteins, which bind to a hydrophobic groove on Bcl-2 and its pro-survival homologs, thereby neutralizing their capacity to prevent activation of Bax or Bak.
Once activated, Bax and Bak permeabilize the outer mitochondrial membrane, releasing cytochrome c to trigger formation of the Apaf-1 scaffold required for activation of caspase-9.
Activation of Bax and Bak requires neutralization of multiple pro-survival proteins (Willis et al, 2006) and while certain BH3-only proteins (eg Bim, Puma) can engage all the pro-survival proteins, others (eg Bad, Noxa) engage only subsets (Chen et al, 2005).
ABT-737
There is now great interest in developing cancer therapeutics that can mimic the action of the BH3 domain by binding to pro-survival proteins to trigger the apoptotic program.
Abbott has reported a very promising BH3 mimetic, ABT-737, which binds strongly to Bcl-2, Bcl-xL and Bcl-w, but not to Mcl-1 or A1 (Oltersdorf et al, 2005). While very effective against many follicular lymphomas, chronic lymphocytic leukemias and small cell lung cancers, most other tumors were refractory.
Resistance to ABT-737 was shown to correlate with expression of Mcl-1 and its downregulation by a variety of strategies conferred sensitivity (van Delft et al, 2006).
In vivo studies in a mouse lymphoma model suggest that this drug should be efficacious in tumors with low Mcl-1 levels or when combined with agents that inactivate Mcl-1, even in the face of Bcl-2 over-expression (van Delft et al, 2006; Mason et al, unpublished).
Targeted therapy
Animal models
Proof of principle is provided by models in which mice overexpressing BCL2 protein developed follicular hyperplasia and extended survival of B lymphocytes; conversely, mice deficient for a proapoptotic BCL2 family member, BAD, developed DLBCL of GC origin.
References
Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008 Jan;9(1):47-59. PMID: 18097445
Abramson JS, Shipp MA. Advances in the biology and therapy of diffuse large B-cell lymphoma: moving toward a molecularly targeted approach. Blood. 2005 Aug 15;106(4):1164-74. PMID: 15855278
Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002 Sep;2(9):647-56. PMID: 12209154
Immunohistochemical studies of pediatric intestinal pseudo-obstruction: bcl2, a valuable biomarker to detect immature enteric ganglion cells. Park SH, Min H, Chi JG, Park KW, Yang HR, Seo JK. Am J Surg Pathol. 2005 Aug;29(8):1017-24. PMID: 16006795