Home > D. General pathology > Infectious diseases > Epstein-Barr virus
Epstein-Barr virus
Tuesday 10 June 2003
Definition: Epstein Barr virus (EBV) is a ubiquitous herpes virus (formally human herpes virus 4) that infects more than 90% of adults worldwide. In the United States, it is estimated that greater than 80% of individuals by age 19 are seropositive for EBV.
Images
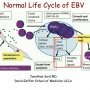
Normal life cycle of EBV
EBV is generally transferred though saliva. Primary infection can occur throughout life. If the primary infection occurs early in life, there may be few or no symptoms; however, if the primary infection occurs later symptoms of infectious mononucleosis may occur.
EBV is the first virus linked to malignancies, both epithelial and lymphoid. Malignant transformation of B cells infected with EBV result in EBV associated lymphomas including T and B cell non Hodgkin lymphoma as well as Hodgkin lymphoma.
Because EBV is associated with malignancy in only a subset of EBV infected individuals, there may be an abnormal immune response against the virus which allows EBV associated malignant lymphomas to develop.
Digital cases
HPC:193 : Infectious mononucleosis
HPC:333 : EBV-associated infectious mononucleosis
Pathology
EBV-associated diseases
- EBV-associated hemophagocytic syndrome (12481907, 12467966, 11456362, 8389318)
- EBV-associated infectious mononucleosis
EBV-associated tumors
- EBV-associated lymphoproliferations
- EBV-associated lymphomas
- EBV-associated carcinomas
- EBV-associated smooth muscle tumors
Pathogenesis
EBV is transmitted by close human contact, frequently with the saliva during kissing. An EBV envelope glycoprotein binds to CD21 (CR2), the receptor for the C3d component of complement, present on B cells. The viral infection begins in nasopharyngeal and oropharyngeal lymphoid tissues, particularly the tonsils.
Although EBV has the capacity to infect epithelial cells (mainly through binding to the integrin α5/β1), the role of epithelial infection in acute mononucleosis (and long-term viral shedding) remains uncertain.
Either through transient infection of epithelium or transcytosis into the submucosa, EBV gains access to sub-mucosal lymphoid tissues. Here, infection of B cells may take one of two forms. In a minority of B cells, there is productive infection with lysis of infected cells and release of virions, which may infect other B cells.
In most B cells, EBV establishes latent infection. Of note, patients with X-linked agammaglobulinemia, who lack B cells, do not become latently infected with EBV or shed virus, suggesting B cells are the main reservoir of latent infection.
At least 11 EBV genes are involved in the establishment of latency, including EBNA1, which plays a role in EBV DNA replication, and EBNA2 and latent membrane protein-1 (LMP-1), which drive B cell activation and proliferation.
LMP-1 appears to act by binding to TNF receptor-associated factors (TRAFs), and activates signaling pathways that mimic B-cell activation by the TNF receptor homologue, CD40, which is involved in normal B-cell responses. EBNA-2 stimulates transcription of many host cell genes, including cyclin D, which regulates cell cycle progression.
The activated B cells then disseminate in the circulation and secrete antibodies with several specificities, including the heterophile anti-sheep red blood cell antibodies used for the diagnosis of infectious mononucleosis. Heterophile antibodies bind to antigens thought to differ from the antigens that induced them.
People with mononucleosis make antibodies that agglutinate sheep or horse red blood cells in the laboratory, but these antibodies do not react with EBV.
The symptoms of infectious mononcleosis appear upon initiation of the host immune response. Cellular immunity mediated by cytotoxic CD8+ T cells and natural killer cells is the most important component of this response.
The atypical lymphocytes seen in the blood, so characteristic of this disease, are mainly CD8+ cytotoxic T cells, but also include CD16+ NK cells. The reactive proliferation of T cells is largely centered in lymphoid tissues, which accounts for the lymphadenopathy and splenomegaly.
Early in the course of the infection, IgM antibodies are formed against viral capsid antigens; later, IgG antibodies are formed that persist for life. In otherwise healthy persons, the fully developed humoral and cellular responses to EBV act as brakes on viral shedding, resulting in the elimination of B cells expressing the full complement of EBV latency-associated genes.
However, EBV persists throughout life in a small population of resting B cells in which expression of EBV genes is limited to EBNA1 and latent membrane protein 2.
Cells within this pool are thought to occasionally reactivate expression of the other latency-associated genes, such as EBNA2 and LMP1, causing them to proliferate. Particularly in hosts with acquired defects in cellular immunity (e.g., AIDS), this proliferation can progress through a multi-step process to EBV-associated B cell lymphomas.
One example is Burkitt lymphoma, in which a chromosomal translocation (most commonly an 8:14 translocation) involving the c-myc oncogene is a critical additional oncogenic event.
Morphology
The major alterations involve the blood, lymph nodes, spleen, liver, central nervous system, and, occasionally, other organs. The peripheral blood shows absolute lymphocytosis with a total white cell count between 12,000 and 18,000 cells/μl, more than 60% of which are lymphocytes. Many of these are large, atypical lymphocytes, 12 to 16 μm in diameter, characterized by an abundant cytoplasm containing multiple clear vacuolations, an oval, indented, or folded nucleus, and scattered cytoplasmic azurophilic granules (Fig. 8-17). These atypical lymphocytes, most of which express CD8, are usually sufficiently distinctive to permit the diagnosis from examination of a peripheral blood smear.
The lymph nodes are typically discrete and enlarged throughout the body, principally in the posterior cervical, axillary, and groin regions. On histologic examination, the most striking feature is the expansion of paracortical areas by activated T cells (immunoblasts). A minor population of EBV-infected B cells expressing EBNA2, LMP-1, and other latency-specific genes can also be detected in the paracortex using specific antibodies. Occasionally, EBV-infected B cells resembling Reed-Sternberg cells may be found. B cell areas (follicles) may also be hyperplastic, but this is usually mild in degree. The T cell proliferation is sometimes so exuberant that it is difficult to distinguish the nodal morphology from that seen in malignant lymphomas. Similar changes
The spleen is enlarged in most cases, weighing between 300 and 500 gm. It is usually soft and fleshy, with a hyperemic cut surface. The histologic changes are analogous to those of the lymph nodes, showing an expansion of white pulp follicles and red pulp sinusoids due to the presence of numerous activated T cells. These spleens are especially vulnerable to rupture, possibly in part because the rapid increase in size produces a tense, fragile splenic capsule.
Liver function is almost always transiently impaired to some degree, although hepatomegaly is at most moderate. On histologic examination, atypical lymphocytes are seen in the portal areas and sinusoids, and scattered, isolated cells or foci of parenchymal necrosis filled with lymphocytes may be present. This histologic picture may be difficult to
The central nervous system may show congestion, edema, and perivascular mononuclear infiltrates in the leptomeninges. Myelin degeneration and destruction of axis cylinders have been described in the peripheral nerves.
Although infectious mononucleosis classically presents with fever, sore throat, lymphadenitis, and the other features mentioned earlier, often its behavior is atypical.
It may present with little or no fever and only malaise, fatigue, and lymphadenopathy, raising the specter of leukemia or lymphoma; as a fever of unknown origin without significant lymphadenopathy or other localized findings; as hepatitis that is difficult to differentiate from one of the hepatotropic viral syndromes; or as a febrile rash resembling rubella.
Ultimately, the diagnosis depends on the following findings (in increasing order of specificity): (1) lymphocytosis with the characteristic atypical lymphocytes in the peripheral blood, (2) a positive heterophile antibody reaction (monospot test), and (3) specific antibodies for EBV antigens (viral capsid antigens, early antigens, or Epstein-Barr nuclear antigen).
In most patients, infectious mononucleosis resolves within 4 to 6 weeks, but sometimes the fatigue lasts longer.
One or more complications occasionally supervene. They may involve virtually any organ or system in the body. Perhaps most common is marked hepatic dysfunction with jaundice, elevated hepatic enzyme levels, disturbed appetite, and rarely even liver failure.
Other complications involve the nervous system, kidneys, bone marrow, lungs, eyes, heart, and spleen (splenic rupture has been fatal). A more serious complication in those suffering from some form of immunodeficiency, such as AIDS, or receiving therapy that leads to defects of cellular immunity (e.g., bone marrow or solid organ transplant recipients) is that the B-cell proliferation may run amok, leading to death.
In AIDS, this usually takes the form of monoclonal B-cell lymphomas, whereas in the setting of acute severe immunosuppression (e.g., bone marrow transplantation), even polyclonal proliferations may prove fatal.
X-linked lymphoproliferation syndrome (XLP)
These unfortunate consequences also occur in individuals suffering from the X-linked lymphoproliferation syndrome (XLP, also known as Duncan disease), a disorder caused by a defect in a gene, SH2D1A, that is expressed primarily in cytotoxic T cells and NK cells.73 SH2D1A (also called SAP) participates in a signaling pathway critical for an effective cellular response to EBV-infected B cells. Patients are often normal until they are acutely infected with EBV, often during adolescence.
The failure to control EBV infection variously leads to chronic infectious mononucleosis, agammaglobulinemia, and B cell lymphoma, each of which proves fatal in about a third of patients.
References
Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004 Mar 25;350(13):1328-37. PMID: 15044644
Paludan C, Munz C. CD4+ T cell responses in the immune control against latent infection by Epstein-Barr virus. Curr Mol Med. 2003 Jun;3(4):341-7. PMID: 12776989
Ambinder RF, Mann RB. Detection and characterization of Epstein-Barr virus in clinical specimens. Am J Pathol. 1994 Aug;145(2):239-52. PMID: 8053485