Home > G. Tumoral pathology > yolk sac tumor
yolk sac tumor
Tuesday 14 October 2003
endodermal sinus tumor, yolk sac tumors
Definition: The yolk sac tumor is a malignant germ cell neoplasm that recapitulates the primary embryonic yolk sac tissue (endodermal sinus).
Digital case
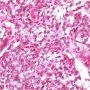
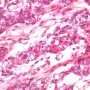
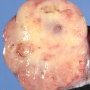
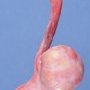
JRC:18783 : Yolk sac carcinoma arising in liver
Images
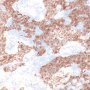
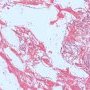
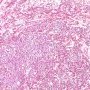
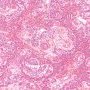
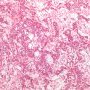
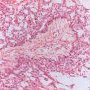
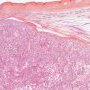
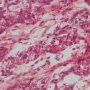
Yolk sac tumors
- https://twitter.com/HenryTranMD/status/715457178912428032
- https://twitter.com/Konzult_/status/830858117973880832
Localization
ovarian yolk sac tumor
testicular yolk sac tumor
YST is the most common malignant testicular germ cell tumor of pediatric population (60 to 70%). Intratubular neoplasia (ITGCN) is absent.
The yolk sac tumor is the third most common form of ovarian germ cell tumor (approx 1% of cases) and is a common component of mixed germ cell tumors of the testis.
Most of the ovarian yolk sac tumors occur as pure neoplasms, whereas pure yolk sac tumors of the testis are rare in adults but the most common testicular germ cell tumor in children, peaking at 1.5 years of age and representing about 70% of pediatric testicular germ cell tumors.
There are numerous patterns of yolk sac tumor, beautifully described and illustrated by Teilum in his classical writings on this tumor, and only a few of the more challenging ones will be discussed here, some of them recognized since Dr Teilum’s death. Often these are admixed with other patterns that facilitate the diagnosis.
One of the classic errors in tumor pathology is the misinterpretation of clear cell carcinoma of the ovary as yolk sac tumor. For years both these tumors were considered the same entity until Teilum resolved that issue. Clear cell carcinoma shares with yolk sac tumor tubular, cystic, and papillary patterns, but other distinctive yolk sac tumor patterns are absent; the patients are significantly older than those with yolk sac tumor, and it is not associated with elevated serum alpha-fetoprotein, although, treacherously, it can stain for it immunohistochemically. Only uncommon or deceptive patterns are considered here.
The solid pattern of yolk sac tumor mimics germinoma by virtue of its sheet-like arrangement of lightly staining cells with well-defined borders.
A greater degree of nuclear variability, occasional suggestion of microcysts (although, as mentioned, this feature may also be seen less commonly in germinomas) and scarcity of lymphocytes in solid yolk sac tumor are helpful in making the distinction from germinoma, as is the occasional occurrence of intracytoplasmic hyaline globules and extracellular basement membrane deposits, features lacking in germinomas.
Most helpful is the occurrence of more distinctive yolk sac tumor patterns, which are almost always present in sizable samples but may be absent in limited material, as in biopsies from metastatic sites. Immunostains can assist in problematic cases since cytokeratin (AE1/AE3) and AFP stains are often positive in solid yolk sac tumor and negative in germinoma.
Additionally, the OCT3/4 immunostain is positive in germinoma, but negative in yolk sac tumor.
Rarely there is a solid, blastema-like growth of small cells in yolk sac tumor, but this is always associated with typical patterns in our experience.
Glandular patterns occur in about one-third of yolk sac tumors, usually taking the form of scattered, simple glands and representing no diagnostic problem.
endometrioid-like yolk sac tumor
Rarely, a predominantly or purely glandular pattern occurs in primary yolk sac tumors, almost always in the ovary, often with prominent subnuclear vacuolization, thereby resembling secretory endometrioid carcinoma (endometrioid-like yolk sac tumor).
In cases of testicular germ cell tumor, purely glandular patterns are almost exclusively seen in metastases resected after chemotherapy rather than in the primary tumors. They are most common in recurrences seen two or more years after the initial diagnosis.
Endometrioid-like yolk sac tumor occurs in younger patients (mean, 22 years) than those with endometrioid adenocarcinoma (mean, 56 years) and the latter is frequently associated with endometriosis or an endometrioid adenofibroma.
The frequently elevated serum AFP level in patients with glandular yolk sac tumors contrasts with the normal level in cases of endometrioid adenocarcinomas.
This differential is complicated by the fact that rarely yolk sac tumor arises on the background of a somatic ovarian neoplasm, which is most commonly endometrioid adenocarcinoma, but these tumors occur in an older age group than yolk sac tumors and have a number of helpful pathological distinguishing features, most important of which is the associated conventional carcinoma.
The differential of a purely glandular testicular yolk sac tumor is limited, mainly being with a rare adenocarcinoma that has developed from pre-existing teratoma. Immunostains are helpful in distinguishing adenocarcinoma and glandular yolk sac tumor; AFP is positive, and EMA and cytokeratin 7 are negative in endometrioid-like yolk sac tumor and show opposite reactivities in endometrioid adenocarcinoma.
Hepatoid variant
Hepatoid differentiation, usually scattered, small clusters of polygonal cells with abundant eosinophilic cytoplasm and prominent nucleoli, is common in yolk sac tumors, occurring in 22% of cases in one series and is infrequently seen in other ovarian tumors.
Rarely, there is a predominant or pure hepatoid pattern; in males prominent hepatoid differentiation is almost always seen in metastatic lesions resected after chemotherapy, particularly in the context of late recurrence, rather than in the primary.
For pure or predominantly hepatoid ovarian yolk sac tumors, the differential includes the rare hepatoid carcinoma. The occurrence of the latter in older patients and its common admixture with an adenocarcinomatous component of surface epithelial type (usually serous), sometimes evident only in metastases, are helpful findings.
Distinction of hepatoid yolk sac tumor from metastatic hepatocellular carcinoma to the ovary is usually facilitated by knowledge of tumor in the liver, a history of long-standing liver disease and presence in the ovarian tumor of minor foci of yolk sac neoplasia.
basement membrane deposits
Lastly, yolk sac tumors commonly have abundant basement membrane deposits that surround individual and small groups of tumor cells.
Rarely, this ’parietal’ differentiation (named for the comparable structure in the rodent yolk sac) becomes predominant, leaving isolated tumor cells in a dense basement membrane matrix.
This tends to occur most frequently, at least in testicular cancer patients, in resected metastases following chemotherapy, and it may be misinterpreted as a matrix-producing mesenchymal lesion if the pathologist is not aware of the phenomenon.
Epidemiology
Age: six months to five years, peak in boys of age less than 2 years
LOH
6q21-26 LOH (11753688)
1p36 LOH (1p36 deletion) (11753688)
Pathogenesis
Hypermethylation of the RUNX3 gene promoter.
Pediatric cases express GATA-4, a transcription factor that regulates differentiation and function of murine yolk sac endoderm
Treatment
1. Surgical Management: Radical Orchiectomy with high ligation of the spermatic cord with Retroperitoneal Lymph Node Dissection
- Indications
- a. The presence of a persistent retroperitoneal mass following chemotherapy
- b. Persistent elevation of serum AFP following chemotherapy with no evidence of metastases on imaging studies
- c. Normal or unknown serum AFP levels at time of diagnosis
2. Chemotherapy : Platinum-based regimens seems to apply to childhood germ cell Prognosis Excellent (5 year survival 90% even in advanced cases)
Variants
prepubertal yolk sac tumor
- Isochrome 12p is not seen
- Euploid or tetraploid
- Recurrent nonrandom chromosomal abnormalities: 1p deletion, 3p duplication, 6q deletion
- p53 gene mutations absent.
- Equal incidence of lymphatic and hematogenous spread for metastases.
- Presents as stage I disease (85%)
adult yolk sac tumor
- presents along with other germ cell tumor components.
- Isochrome 12p positive
- Intratubular neoplasia is present.
- Usually aneuploid p53 gene mutations.
- Lymphatic route is primarily spread for metastases.
- Often presents as higher stage disease
Microscopical synopsis
microcystic pattern
- interconnecting cords and ribbons of tumor cells are surrounded by abundant myxoid stroma
- intracellular vacuoles
- vesicular nuclei with punctate nucleoli
- often coexists with other architectural patterns
myxomatous pattern
- neoplastic stellate cells
- spindle cells
- epithelioid cells
- abundant myxoid stroma
- many of these cells are pluripotential and can form skeletal muscle, cartilage, and bone (such areas should not be confused with teratoma)
endodermal sinus pattern
- Schiller-Duvall body: central vessel surrounded by tumor cells in a cystic space often lined by flattened tumor cells
hepatoid pattern (20%)
- sheets of polygonal cells with abundant eosinophilic cytoplasm
- hyaline globules
- bile canaliculi
solid pattern
- sheets of uniform tumor cells with clear or pale pink cytoplasm
- differential diagnosis: seminoma (it lacks the fibrous septa with lymphoid infiltrate seen in seminoma)
Cytogenetics
i(12p) in adults and children (8522320)
deletion of chromosome 1p36 (9162192)
deletion of distal regions of chromosome 6q
Molecular biology
LOH of 6q (72%) (11753688)
LOH of 1p (45%) (11753688)
Differential diagnosis
embryonal Carcinoma
mature teratoma and immature teratoma
Sertoli cell tumor
seminoma
Case records
[Case HP #14827->14827]
References
Yolk Sac Tumor of the Testis in Infants and Children: A Clinicopathologic Analysis of 33 Cases. Cornejo KM, Frazier L, Lee RS, Kozakewich HP, Young RH. Am J Surg Pathol. 2015 Mar 30. PMID: 25828390
Deletion mapping of 6q21-26 and frequency of 1p36 deletion in childhood endodermal sinus tumors by microsatellite analysis. Hu J, Schuster AE, Fritsch MK, Schneider DT, Lauer S, Perlman EJ. Oncogene. 2001 Nov 29;20(55):8042-4. PMID: 11753688
Detection of chromosome aberrations in paraffin sections of seven gonadal yolk sac tumors of childhood. Jenderny J, Köster E, Meyer A, Borchers O, Grote W, Harms D, Jänig U. Hum Genet. 1995 Dec;96(6):644-50. PMID: 8522320