Home > G. Tumoral pathology > germinoma
germinoma
Tuesday 6 June 2006
Digital cases
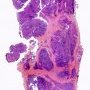
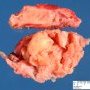
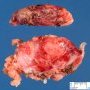
JRC:1231 : Mediastinum, anterior: Germinoma with multilocular thymic cyst .
JRC:19001 : Mediastinum, anterior: thymic germinoma.
JRC:19005 : Thymic seminoma with marked cystic changes, superior mediastinum.
JRC:7861 : Mediastinum: Germinoma with yolk sac component.
JRC:7851 : Mediastinum: Germinoma (embryonal).
JRC:7852 : Mediastinum: Germinoma.
JRC:7853 : Mediastinum: Germinoma.
JRC:7862 : Mediastinum: Germinoma.
Definition: Seminoma is the most common testicular germ cell tumor, representing 50% of the cases, and dysgerminoma, the second most common ovarian germ cell tumor, although it represents only about 2% of them because of the marked predominance of teratomas in the ovary compared to their much lesser frequency in the testis.
This tumor is essentially morphologically identical in the two gonads, and so this discussion considers some of the diagnostic problems posed by seminoma and dysgerminoma together.
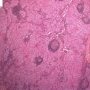
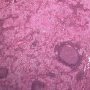
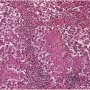
The classic pattern of germinoma is distinctive and readily recognized based on its overall sheet-like arrangement of clear cells with well-defined cytoplasmic borders (in well-fixed specimens) and flattened, ’squared-off’ nuclear membranes that is subdivided into variably sized, smaller groups of cells (alveolar aggregates, nests, clusters) by lymphocyte-bearing, fibrovascular septa.
There are, however, some unusual patterns that are prone to misinterpretation. Occasional germinomas have a microcystic or cribriform arrangement that may suggest yolk sac tumor. This finding is more common in seminoma than dysgerminoma. This change may, in part, be secondary to edema because some examples of this phenomenon have faintly eosinophilic fluid within the cystic spaces, but in other examples this feature is lacking.
Some cases have few lymphocytes, further complicating the interpretation since a lymphocytic infiltrate is a usual feature of typical germinoma, occurring in almost all of them, and its presence is a helpful criterion for the diagnosis of germinoma, whereas yolk sac tumor generally lacks lymphocytes.
Helpful differential features include that the cystic spaces in these unusual cases of germinoma frequently contain exfoliated tumor cells or inflammatory cells and are lined by polygonal tumor cells rather than the flattened lining cells with compressed nuclei of microcystic yolk sac tumor.
The key differential findings, however, are retention of the usual cytological features of germinoma cells and absence of other patterns that would be expected in yolk sac tumor. Immunostains for cytokeratin (AE1/AE3), alpha-fetoprotein (AFP), and OCT3/474 are helpful, typically staining negatively (AE1/AE3 and AFP) and positively (OCT3/4) in germinoma and showing opposite reactivities in yolk sac tumor.
OCT3/4 (also known as POU5F1) is a newly described nuclear transcription factor that is expressed in human embryonic and stem cells and has been found to mark the nuclei of germinomas and embryonal carcinomas with very high sensitivity and specificity, being positive in all 189 examples of these tumors in two studies and reactive in no other form of invasive germ cell tumor or other form of primary testicular tumor.
Additionally, among 3439 tumors studied with tissue microarrays, apart from germinoma, embryonal carcinoma, and gonadoblastoma, only three other tumors stained positively (one of 50 clear cell carcinomas of the kidney, one of 50 squamous cell carcinomas of the lung and one of 47 large cell carcinomas of the lung).
Occasional germinomas have a solid tubular pattern characterized by elongated nests with a somewhat palisaded arrangement of tumor cells at their periphery.
This may cause confusion with Sertoli cell tumor, but adjacent intratubular germ cell neoplasia of the unclassified type (IGCNU) may be helpful, if present, and more reliably the nuclei have the typical appearance of germinoma nuclei.
When the light microscopic features are ambiguous, immunostains for placental alkaline phosphatase (PLAP) and OCT3/4 (positive in germinoma and negative in Sertoli cell tumor) and inhibin (negative in germinoma and often positive in Sertoli cell tumor) are helpful.
Focal intertubular growth is common in seminoma, and rarely characterizes most of, or the entire, tumor. Seminomas with purely intertubular growth may not produce a clinical mass, and the tumor is often heralded by metastases or found during the investigation of infertility. The neoplastic infiltrate in these cases may be deceptively subtle and potentially overlooked at scanning magnification. The seminiferous tubules remain intact with, in some cases, expansion of the space between them if the tumor cells are sufficiently numerous. In some cases, however, the seminoma cells subtly infiltrate the interstitium as individual cells or small clusters without appreciable intertubular expansion.
They may be admixed with clusters of Leydig cells, which may further mask their presence. Clues to this process include the common associated lymphocytic infiltrate in the interstitium (although this may be misinterpreted as a manifestation of viral orchitis), the usual presence of IGCNU and the frequently associated testicular atrophy and Leydig cell hyperplasia.
Because lymphomas and metastases may show prominent intertubular growth, it is important to identify the cytological features of seminoma cells in these cases to rule out other neoplastic infiltrates. Appropriate immunostains can easily resolve the differential if necessary.
Germinomas may have areas with cells that have denser, sometimes eosinophilic or amphophilic cytoplasm and increased nuclear crowding and irregularity; this appearance must be distinguished from embryonal carcinoma.
Frequently, these findings relate to suboptimal fixation of germinoma, resulting in poorly defined cell membranes, cytoplasmic autolysis and secondary nuclear crowding, but occasionally they occur as real phenomenon in well-processed material.
Some germinomas may have these features diffusely, yielding an overall plasmacytoid appearance. In the past, many such testicular cases were considered ’anaplastic’ seminomas, a term that often causes confusion in the minds of pathologists and oncologists alike and which is not recommended.
Tickoo et al identified a number of seminomas that had these histological features and that also tended to stain more prominently for cytokeratin and CD30 than the usual seminoma. They found that these ’atypical’ seminomas tended to present at a higher tumor stage and advocated that they may merit more aggressive treatment. Others, myself included, however continue to place these cases into the seminoma category without separately designating them as atypical since it is not clear that different treatment is indicated.
On most occasions the diagnosis of embryonal carcinoma is straightforward, but small foci within a background of germinoma may be challenging to identify.
Any distinct epithelial differentiation, gland or papilla formation, should be regarded as evidence of embryonal carcinoma.
In the absence of distinct epithelial features, a combination of findings (nuclear pleomorphism, nuclear crowding and irregularity, dense cytoplasm, indistinct cell borders) will cause concern for transformation of germinoma to embryonal carcinoma.
For those borderline cases where there is ambiguity at the routine light microscopic level, immunostains for CD30 and cytokeratin (AE1/AE3) may be useful.82 Positivity in the problematic areas for these markers that contrasts with negativity in the typical germinoma areas provides support for an embryonal carcinoma component.
If the borderline findings are diffuse, impressive AE1/AE3 and CD30 reactivity should be required, rather than focally prominent positivity for one of these markers, before accepting the tumor as embryonal carcinoma.
About 5% of germinomas have distinct admixed syncytiotrophoblast cells, although additional cases with inconspicuous syncytiotrophoblast cells can be identified using immunohistochemical stains for human chorionic gonadotropin (hCG).
When these cells are widely dispersed, as is typically the case, this phenomenon does not usually create diagnostic confusion. However, when they occur in sizable clusters, there may be concern for choriocarcinoma.
Unlike choriocarcinoma, however, these tumors lack the plexiform admixture with cytotrophoblast cells, which show a greater degree of pleomorphism than the uniform, germinoma cells associated with admixed syncytiotrophoblast cells. Additionally, other areas of the tumor have the typical findings of germinoma.
Syncytiotrophoblast cells in germinoma, just as in choriocarcinoma and other types of germ cell tumor, synthesize and secrete hCG, which may, therefore, cause a number of hormonal manifestations.
These include androgenic ones from secondary hyperplasia of Leydig cells in the testis or stimulation of the ovarian stroma; estrogenic ones, either because of direct stimulation of ovarian stroma and/or non-neoplastic follicles or peripheral conversion of androgen to estrogen, thereby resulting in abnormal uterine bleeding or, in men, gynecomastia; and hyperthyroidism because of the thyroid-stimulating hormone-like activity of hCG.
A number of other interesting, paraneoplastic manifestations may be seen in association with germ cell tumors, especially germinomas, including hypercalcemia, hypoglycemia, exophthalmos, autoimmune hemolytic anemia (usually ovarian dermoids), ataxia telangiectasia, and limbic encephalopathy.
Localization
mediastinal seminoma
- thymic seminoma
testicular seminoma (seminoma of the testis)
Differential diagnosis
yolk sac tumors (15767805)
See also
germ cell tumors
- dysgerminoma
- spermatocytic seminoma
References
Ulbright TM, Young RH. Seminoma with tubular, microcystic, and related patterns: a study of 28 cases of unusual morphologic variants that often cause confusion with yolk sac tumor. Am J Surg Pathol. 2005 Apr;29(4):500-5. PMID: 15767805