Home > E. Pathology by systems > Digestive system > Esophagus > epithelial dysplasia in Barrett esophagus
epithelial dysplasia in Barrett esophagus
Wednesday 15 February 2017
See also : Barrett esophagus
Types
low-grade dysplasia in Barrett esophagus
high-grade dysplasia in Barrett esophagus
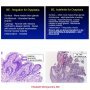
Images
"Crypt dysplasia" of BE-atypical with lots of mitotic activity, large rounded nuclei, apoptosis. P53
High grade dysplasia in Barrett esophagus with strong nuclear TP53 immunolabeling
*https://twitter.com/ARP_Press/status/847587416995016704
Classification
Barrett’s Esophagus & Dysplasia : Tips
Dysplasia is an imperfect marker of cancer risk, but is still the best we have
Most biopsies are NEGATIVE FOR DYSPLASIA
Most patients never progress to dysplasia/cancer
Low magnification is often most useful in dysplasia recognition
Dysplasia is recognizable at low magnification (hyperchromatic)
"Baseline atypia" of Barrett’s mucosa (regenerative zone)
Hold out for cytologic atypia on surface epithelium
Be wary of active inflammation
Don’t use "indefinite for dyspiasia" as a crutch
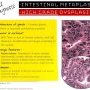
BE - Negative for Dysplasia
Surface - More mature than glands Architecture - Abundant lamina propria
Cytology - Normal with mitoses confined to deeper glands. Nuclei with smooth nuclear membranes. Normal nuclear polarity
No abrupt transition
Inflammation - Variable
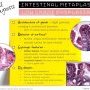
BE, Indefinite for Dysplasia
Surface — often more mature than glands Architecture - slight glandular crowding Cytology - hyperchromasia, nuclear membrane irregularities, increased mitoses in deep glands. Maintained nuclear polarity
Inflammation - Frequently a factor
Nice to see an abrupt transition to be sure somethinq is dysplastic — and thus clonal
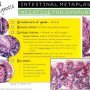
BE, Low grade dysplasia
Clearly neoplastic
Minimal loss of nuclear polarity
Surface involved
Only mild architectural crowding
BE - High Grade dysplasia
- Surface - No maturation Architecture - Crowded glands overrunning lamina propria
Cytology - Nuclear membrane irregularities, extending to surface and loss of nuclear polarity
Inflammation - Typically not abundant
BE - Intramucosal Carcinoma
Surface - No maturation
Architecture - Effacement of lamina propria and syncytial growth pattern of glands. Back-to-back microglands, "dirty necrosis" in glands,
DESMOPLASIA not yet developed
Cytology - as in HG —but often with nucleoli
Inflammation - variable
p53 IHC
p53 is the most frequently mutated gene in human cancer, and it is central to the progression of Barrett’s oesophagus to cancer.
Abnormal p53 expression is predictive of progression of Barrett’s to cancer and provides a helpful adjunct to the sometimes problematic diagnosis of dysplasia.
p53 immunostaining in Barrett’s oesophagus (BO) has been shown to be predictive of progression, but data regarding its generalizability to routine practice are lacking.
p53 immunohistochemistry interpretation is more reliable than dysplasia diagnosis, even with limited training.
As it is predictive of prognosis and improves diagnostic reproducibility, it is suitable for routine use by pathologists as an adjunct to dysplasia diagnosis.
The distinction of LGD versus HGD is poor.
Open references
Diagnosis and grading of dysplasia in Barrett’s oesophagus. Odze RD. J Clin Pathol. 2006 Oct;59(10):1029-38. PMID: 17021130 Free
Paywall references
Barrett’s dysplasia and the Vienna classification: reproducibility, prediction of progression and impact of consensus reporting and p53 immunohistochemistry. 2009. doi : 10.1111/j.1365-2559.2009.03288.x
Dysplasia in Barrett’s oesophagus: p53 immunostaining is more reproducible than haematoxylin and eosin diagnosis and improves overall reliability, while grading is poorly reproducible. 2016. doi : 10.1111/his.12956
A study of indefinite for dysplasia in Barrett’s oesophagus: reproducibility of diagnosis, clinical outcomes and predicting progression with AMACR (α-methylacyl-CoA-racemase). 2010. doi : 10.1111/j.1365-2559.2010.03571.x
p53 Immunohistochemistry as a biomarker of dysplasia and neoplastic progression in Barrett’s oesophagus. 2015. doi : 10.1016/j.mpdhp.2015.04.001