Home > E. Pathology by systems > Digestive system > Esophagus > Barrett esophagus
Barrett esophagus
Wednesday 29 October 2003
Barrett’s oesophagus, Barrett oesophagus, Barrett’s esophagus; Barrett mucosa; Barrett metaplasia; metaplastic endobrachyesophagus
See also : endobrachyesophagus
Images
PAS and Alcian Blue stain in Barrett esophagus
http://www.webpathology.com/case.asp?case=185
adenocarcinoma in Barrett esophagus mucosa
Links
at Johns Hopkins Pathology
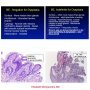
Definition: Barrett esophagus (BE) is defined by the presence of metaplastic esophageal columnar epithelium with goblet cells within endoscopically recognizable areas of the esophagus. Barrett esophagus is a complication of long standing gastroesophageal reflux disease (GERD).
Barrett’s esophagus is defined by metaplastic glandular changes to the distal esophagus and is linked to an increased risk of esophageal adenocarcinoma. As a result of metaplastic changes, the distal esophagus shows areas lined by specialized columnar epithelium with goblet cells.
Controversy exists whether the definition should be limited to intestinal type glands with goblet cells or should be expanded to include non-goblet cell columnar epithelium.
For some, Barrett esophagus refers to the presence of intestinal metaplasia within esophageal squamous mucosa above the level of lower esophageal sphincter.
For these authors, the diagnosis of Barrett esophagus requires both the recognition of abnormal areas on endoscopy as well as histologic evidence of intestinal metaplasia.
The current definition for Barrett’s esophagus (BE) proposed by the American Gastroenterological Association (AGA) is : “the condition in which any extent of metaplastic columnar epithelium that predisposes to cancer development replaces the stratified squamous epithelium that normally lines the distal esophagus”.
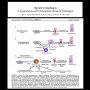
Barrett’s esophagus can be associated with extensive instability and clonal dynamics that evolve from an initial stage characterized by small recombination-based alterations to one with larger copy change events likely associated with mitotic instability.
Barrett’s esophagus may be asymptomatic in a large proportion of the population but screening should be considered for those with certain clinical findings.
The diagnosis of Barrett’s should be based on the combination of careful endoscopic evaluation and histologic review of the biopsy material.
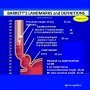
Barrett esophagus is a complication of long standing gastroesophageal reflux disease (GERD). Barrett esophagus is found in almost 10% of individuals with symptomatic GERD. The typical patient is a white male between the ages of 40 and 60. It may also be seen in children with cystic fibrosis.
On endoscopy, it appears as sharply demarcated areas of red, velvey mucosa extending proximally from the gastroesophageal junction.
Dysplasia
Dysplasia in Barrett esophagus has been recognized to be morphologically heterogenous, featuring adenomatous, foveolar, and hybrid phenotypes.
Continued surveillance biopsies may be necessary in cases of indeterminate or low grade dysplasia. Clinical follow-up of patients with high grade dysplasia should be tailored to the individual patient.
Development of newer endoscopy techniques including chemoendoscopy, chromoendoscopy and use of biomarkers on frozen tissue have shown some promise of identifying patients at risk for malignancy.
Definition
The current definition for Barrett’s esophagus (BE) proposed by the American Gastroenterological Association (AGA) is : “the condition in which any extent of metaplastic columnar epithelium that predisposes to cancer development replaces the stratified squamous epithelium that normally lines the distal esophagus”.
Three types of columnar epithelium are seen in the setting of BE:
(I) gastric-fundic type,
(II) cardia-type,
(III) intestinal-type including goblet cells.
However, only the last type has been clearly linked to an increased risk of malignant progression, with a reported annual risk of esophageal adenocarcinoma (EAC) of about 0.5% per year in patients with intestinal metaplasia of the esophagus .
For this reason, both the AGA and the American College of Gastroenterology (ACG) currently recommend that although columnar-type mucosa can be recognized during endoscopy, the presence of intestinal metaplasia must be confirmed by biopsy before rendering a diagnosis of BE.
The American definition is used in most parts of the world, however, Great Britain and Japan allow the diagnosis of BE to be assigned if only cardiac-type metaplasia is seen on biopsy.
While some advocate the universal adoption of the less stringent criteria, the evidence to do so is controversial.
Gatenbyet al. and Keltyet al. each conducted studies that showed a similar risk of EAC in patients having columnar metaplasia of the esophagus with and without goblet cells.
In contrast, two large population studies from Northern Ireland showed a clear increased risk of cancer when intestinal metaplasia was present versus when only columnar cell change was identified.
A study by Takuboet al. which examined the mucosa adjacent to EAC treated with endoscopic mucosal resection found that most (>70%) were bordered by cardiac-type mucosa rather than intestinal-type mucosa and that 56% had no intestinal-type mucosa in any areas of the resection specimens. They concluded that there is a relationship between EAC and cardiac-type mucosa and that a background of intestinal metaplasia may not be a necessary pre-requisite to EAC.
Two similar studies by Chandrasoma and colleagues had different findings. The first, which examined esophagogastrectomy specimens resected due to adenocarcinoma, showed cardiac mucosa adjacent to all tumors but also showed residual intestinal metaplasia in 65% of cases overall and in 100% of intramucosal tumors as well as those less than 1 cm in diameter.
The second study reviewed two groups:
(I) cases with visible columnar metaplasia of the esophagus which underwent systematic 4-quadrant biopsies every 1 to 2 cm
(II) cases of dysplasia or EAC which did not receive systematic biopsy.
They found that when systematic biopsy was performed, intestinal metaplasia was identified in >87% of cases including all cases with dysplasia or EAC. None of the cases with cardiac type epithelium alone had dysplasia or EAC. In the group which did not receive systematic biopsy but did have dysplasia or EAC, many showed only tumor on biopsy. However, slightly more than half (56%) of those with non-tumor mucosa had residual intestinal-type metaplasia.
They hypothesize that the absence of residual intestinal metaplasia immediately adjacent to many cases of EAC is due to tumor overgrowth and inadequate sampling rather than a true absence. They also propose that when metaplastic columnar epithelium is adequately and systematically biopsied, patients without intestinal metaplasia have a negligible risk of dysplasia and cancer.
Recent data shows that columnar cell epithelium may have an intestinal-type immunohistochemical profile even when goblet cells are not identified.
Various studies have shown significantly increased positivity for intestinal markers such as DAS-1 (14-16), CDX-2 (14,17,18), and HepPar1 (19) as well as a similar cytokeratin (16,20) and mucin (20) expression profile in both goblet cell and non-goblet cell columnar epithelia, which suggests a similar origin.
There have also been studies showing similar molecular alterations in both non-goblet cell and intestinal-type metaplasia including chromosomal instability (21,22), microsatellite instability (22), and similar DNA content abnormalities (23).
Despite the similar phenotypic and molecular profiles, the natural history of columnar cells and goblet cells is not always the same (24) suggesting that additional factors are required for progression toward dysplasia and cancer.
Expanding the definition of BE to include all patients with columnar metaplasia of the esophagus would have substantial societal and personal economic impact. Studies from both the United States and Sweden show that the population of patients with columnar metaplasia of the esophagus without goblet cells is significantly greater than the population with intestinal metaplasia (25,26).
Conducting surveillance on all of these patients has the potential to overwhelm healthcare resources and greatly increase treatment costs.
Also, despite data which demonstrate a normal life expectancy in patients with BE, the cost of life insurance is substantially increased and availability of health insurance is decreased in patients with this diagnosis.
Until such a time as columnar cell metaplasia of the esophagus without goblet cells is clearly shown to convey increased risk of EAC, it seems appropriate to hold back from labeling these patients with BE.
Dysplasia in Barrett esophagus
Dysplasia in Barrett esophagus is dividied into low-grade and high-grade. Dysplastic changes are most often seen in the areas of incomplete intestinal metaplasia.
In high-grade dysplasia, the glands are crowded and irregularly shaped and there is nuclear hyperchromasia, increased mitotic activity, and prominent nucleoli. The glands are irregular in shape. There is marked variation in nuclear size and shape with piling up of nuclei. There is nuclear hyperchromasia, high N:C ratios, prominent nucleoli and increased mitotic activity as well. When viewed at low magnification, the dysplastic areas stand out due to their basophilia and loss of mucus.
High-grade dysplastic changes include nuclear hyperchromatism, irregularity in nuclear size and shape, nuclear stratification, prominent nucleoli, and increased mitotic activity.
Dysplastic changes, in the absence of carcinoma, are found in between 5% to 10% of cases of Barrett esophagus. It increases the risk of developing invasive carcinoma by 30 to 40 times that in the general population.
Treatment options for high-grade dysplasia or intramucosal carcinoma in this setting include esophagectomy, photodynamic therapy, laser ablation, and endoscopic mucosal resection.
CDX2
Studies have suggested a tumor suppressor role for CDX-2 in the metaplasia-dysplasia-carcinoma sequence.
CDX-2 shows gradual downregulation of expression during progression in adenomatous dysplasia but not in foveolar or hybrid dysplasia, supporting a tumor suppressor role, at least in the intestinal pathway.
CDX-2 is also found to be expressed to a greater degree in intestinal metaplasia compared with nonintestinalized columnar metaplasia.
Consistent with CDX-2 as a tumor suppressor, this suggests that nonintestinalized columnar metaplasia may be an unstable intermediate state at risk for neoplastic progression.
Molecular biology
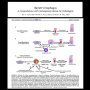
Premalignant Barrett’s esophagus tissue progressed through three molecular stages of disease-CDKN2A(LOH) only, CDKN2A(LOH)/TP53(LOH), and CDKN2A(LOH)/TP53(LOH) with aneuploidy. (17330261)
Increases in both numbers and sizes of regions of LOH or copy number change. In the earliest CDKN2A(LOH) only samples, few regions with both copy change and LOH are detected, whereas copy loss and LOH were highly correlated in more advanced samples. These data indicate that genomic instability increases in severity and changes character during neoplastic progression. (17330261)
See also
Barrett adenocarcinoma
Open references
Barrett’s esophagus: A review of diagnostic criteria, clinical surveillance practices and new developments. Booth CL, Thompson KS. J Gastrointest Oncol. 2012 Sep;3(3):232-42. doi : 10.3978/j.issn.2078-6891.2012.028 PMID: 22943014 [Free]
Bresalier RS. Barrett’s Esophagus and Esophageal Adenocarcinoma. Annu Rev Med. 2008 Sep 10. PMID: 18783330
See also
endobrachyesophagus
Open references
Revised British Society of Gastroenterology recommendation on the diagnosis and management of Barrett’s oesophagus with low-grade dysplasia. 2017. doi : 10.1136/gutjnl-2017-314135
Paywall References
Effects of dropping the requirement for goblet cells from the diagnosis of Barrett’s esophagus. Westerhoff M, Hovan L, Lee C, Hart J.
Clin Gastroenterol Hepatol. 2012 Nov;10(11):1232-6. doi : 10.1016/j.cgh.2012.05.013
PMID: 22642957
Barrett’s Esophagus: A Comprehensive and Contemporary Review for Pathologists. Naini BV, Souza RF, Odze RD.
Am J Surg Pathol. 2016 May;40(5):e45-66. doi : 10.1097/PAS.0000000000000598
PMID: 26813745
Divergent Expression of MUC5AC, MUC6, MUC2, CD10, and CDX-2 in Dysplasia and Intramucosal Adenocarcinomas With Intestinal and Foveolar Morphology: Is This Evidence of Distinct Gastric and Intestinal Pathways to Carcinogenesis in Barrett Esophagus? Khor TS, Alfaro EE, Ooi EM, Li Y, Srivastava A, Fujita H, Park Y, Kumarasinghe MP, Lauwers GY. Am J Surg Pathol. 2012 Mar;36(3):331-342. PMID: 22261707
Barrett’s esophagus: a molecular perspective. Spechler SJ. Curr Gastroenterol Rep. 2005 Jun;7(3):177-81. PMID: 15913475
Intestinal Differentiation in Metaplastic, Nongoblet Columnar Epithelium in the Esophagus. Hahn HP, Blount PL, Ayub K, Das KM, Souza R, Spechler S, Odze RD. Am J Surg Pathol. 2009 Apr 9. PMID: 19363439
Lai LA, Paulson TG, Li X, Sanchez CA, Maley C, Odze RD, Reid BJ, Rabinovitch PS. Increasing genomic instability during premalignant neoplastic progression revealed through high resolution array-CGH. Genes Chromosomes Cancer. 2007 Jun;46(6):532-42. PMID: 17330261
Mino-Kenudson M, Brugge WR, Puricelli WP, Nakatsuka LN, Nishioka NS, Zukerberg LR, Misdraji J, Lauwers GY. Management of Superficial Barrett’s Epithelium-Related Neoplasms by Endoscopic Mucosal Resection: Clinicopathologic Analysis of 27 Cases. Am J Surg Pathol. 2005 May;29(5):680-686. PMID: 15832094
McManus DT, Olaru A, Meltzer SJ. Biomarkers of esophageal adenocarcinoma and Barrett’s esophagus. Cancer Res. 2004 Mar 1;64(5):1561-9. PMID: 14996709
Sarbia M, Donner A, Franke C, Gabbert HE. Distinction between intestinal metaplasia in the cardia and in Barrett’s esophagus: the role of histology and immunohistochemistry. Hum Pathol. 2004 Mar;35(3):371-6. PMID: 15017595
Wild CP, Hardie LJ. Reflux, Barrett’s oesophagus and adenocarcinoma: burning questions. Nat Rev Cancer. 2003 Sep;3(9):676-84. PMID: 12951586
Barrett MT, Sanchez CA, Prevo LJ, Wong DJ, Galipeau PC, Paulson TG, Rabinovitch PS, Reid BJ. Evolution of neoplastic cell lineages in Barrett oesophagus. Nat Genet. 1999 May;22(1):106-9. PMID: 10319873