Home > E. Pathology by systems > Skin > cutaneous fibrous histiocytoma
cutaneous fibrous histiocytoma
Thursday 5 June 2003
Dermatofibroma, Sclerosing hemangioma, Histiocytoma cutis, Nodular subepidermal fibrosis, benign fibrous histiocytoma ; histiocytofibroma
| PO |
Digital cases
UI:923 - benign fibrous histiocytoma (dermatofibroma)
PathConsult
JRC:10461 : Dermatofibroma (fibrous histiocytoma) (Vs. gram cct)
Images
pallisading dermatofibroma
lipidized fibrous histiocytoma
dermatofibroma with basaloid induction
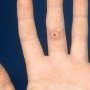
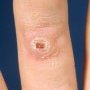
Definition: Benign proliferation of dermal fibroblasts and histiocytes that generally presents as an asymptomatic, variably pigmented nodule, often on the extremities. Type of fibrohistiocytic tumors.
Clinical synopsis
Firm
Nodular
Nonencapsulated
Often pigmented
Chiefly on extremities
Single or multiple
Flat, polypoid or depressed shape
Most @<@1 cm diameter
Some reach huge proportions
Pathogenesis
Longstanding controversy as to whether:
- neoplastic
- favored by occurrence of aggressive and even metastasizing forms
- evidence of clonality3–5
- reactive
Gross Pathology
Usually solid
Rather well circumscribed
Not encapsulated
Colored white to yellow to dark brown depending on relative amounts of:
- fibrous tissue
- fat
- hemosiderin
Histopathology
Characteristically centered in upper dermis
Can involve deep dermis
Occasionally extends into subcutis
Cellular fibroblastic proliferation
Varying amounts of collagen deposition
Variable number of macrophages
- fat (foamy appearance)
- hemosiderin (The tumor is mainly composed of hemosiderin-laden macrophages.)
- some multinucleated cells
- may acquire features of Touton’s giant cells
- more rarely osteoclast-like features with or without bone formation.
Fine vascular network:
- can be prominent
- responsible for: past diagnosis as sclerosing hemangioma
- occasional misdiagnosis as Kaposi’s sarcoma, especially in HIV infection
May be:
- focal storiform features:
- rarely as well developed as in dermatofibrosarcoma protuberans
- smooth muscle proliferation within adjacent dermis
Lesions blend imperceptibly into adjacent dermis
Overlying epidermis:
- normal, atrophic, or acanthotic
- sometimes proliferation of hair germ-like structures in basal layer of epithelium
- This lesion can be associated with basaloid proliferation of the overlying skin. (This change does not represent a basal cell carcinoma)
- rarely basal cell carcinoma develops
- exceptionally squamous cell carcinoma in situ
Morphologic variations (sometimes two or more coexist)
prominent palisading similar to that in peripheral nerve tumors
keloid-like changes
myxoid changes
granular cells
markedly lipidized cells
clear cells
diffuse eosinophilic infiltrate
lichenoid, erosive, and ulcerated features
lipidized fibrous histiocytoma
Variants
marked focal cellular atypia (manifested by ‘monster cells’)
extreme cellularity (sometimes with necrosis)
large cystic changes filled with blood: referred to as hemorrhagic aneurysmal or angiomatoid (distinguish from angiomatoid malignant fibrous histiocytoma seen in deeper sites in younger patients)
epithelioid cell histiocytoma
- mainly large ‘angulated’ epithelioid cells
- notorious for simulating vascular and melanocytic neoplasms
cellular fibrous histiocytoma
aneurysmal fibrous histiocytoma
atypical fibrous histiocytoma (pseudosarcomatous fibrous histiocytoma)
epithelioid dermatofibroma (epithelioid fibrous histiocytoma)
ossifying dermatofibroma with osteoclast-like giant cells
metastasizing "benign" cutaneous fibrous histiocytoma (23426120)
Special Stains and Immunohistochemistry
Proliferating spindle cells:
- positive for: vimentin
- usually negative for: lysozyme, other histiocytic markers (these results raise questions about alleged histiocytic nature)
reactive for:
- FXIIIa: a proenzyme in ‘dermal dendrocytes’
negative for CD34 (in contrast with dermatofibrosarcoma protuberans)
positive for tenascin
Often markers associated with smooth muscle/myofibroblastic differentiation:
- e.g. actin, desmin, myosin
- not generally known; has led to misdiagnoses such as leiomyoma and leiomyosarcoma
Immunochemistry
strong tenascin positivity at the dermal-epidermal junction overlying the lesion (100%)
tenascin within the DF lesion (80%)
CD34+ (25%) DF
Factor XIIIa+ (95%) dermatofibrosarcoma
Differential Diagnosis
melanocytic nevus
Kaposi sarcoma
malignant melanoma
DFSP (11172295)
| Marker | dermatofibroma | DFSP |
| CD34 | 25% | 80% |
| FXIIIa | 95% | 15% |
| Tenascin in the tumor | 80% | 80% |
| Tenascin at the dermal-epidermal junction | 100% | 0% |
When heavily pigmented may be confused with:
- melanocytic nevi
- malignant melanoma
- Kaposi sarcoma
- other vascular tumors
Prognosis
Generally indolent
Local recurrence rare, even if margins inadequate
Rarely:
- locally aggressive
Exceptionally rarely:
- distant metastases
- more common if: in face, deep extension into subcutaneous tissue or cellular fascicles of mitotically active spindle cells
See also
fibrohistiocytic tumors
- angiomatoid fibrous histiocytoma (specific tumoral entity)
References
Metastasizing "benign" cutaneous fibrous histiocytoma: a clinicopathologic analysis of 16 cases. Doyle LA, Fletcher CD. Am J Surg Pathol. 2013 Apr;37(4):484-95. doi : 10.1097/PAS.0b013e31827070d4 PMID: 23426120
Kaddu S, McMenamin ME, Fletcher CD. Atypical fibrous histiocytoma of the skin: clinicopathologic analysis of 59 cases with evidence of infrequent metastasis. Am J Surg Pathol. 2002;26:35–46.
Mentzel T, Kutzner H, Rutten A, Hugel H. Benign fibrous histiocytoma (dermatofibroma) of the face: clinicopathologic and immunohistochemical study of 34 cases associated with an aggressive clinical course. Am J Dermatopathol. 2002;23:419–426.
Calonje E, Fletcher CDM. Cutaneous fibrohistiocytic tumors. An update. Adv Anat Pathol. 1994;1:2–15.
Calonje E. Is cutaneous benign fibrous histiocytoma (dermatofibroma) a reactive inflammatory process or a neoplasm? (Commentary.). Histopathology. 2000;37:278–280.
Chen TC, Kuo T, Chan HL. Dermatofibroma is a clonal proliferative disease. J Cutan Pathol. 2000;27:36–39.
Vanni R, Fletcher CD, Sciot R, Dal Cin P, DeWever I, Mandahl N, et al. Cytogenetic evidence of clonality in cutaneous benign fibrous histiocytomas: a report of the CHAMP study group. Histopathology. 2000;37:212–217.
Zelger B, Sidoroff A, Stanzl U, Fritsch PO, Ofner D, Jasani B, et al. Deep penetrating dermatofibroma versus dermatofibrosarcoma protuberans. A clinicopathologic comparison. Am J Surg Pathol. 1994;18:677–686.
Kamino H, Jacobson M. Dermatofibroma extending into the subcutaneous tissue. Differential diagnosis from dermatofibrosarcoma protuberans. Am J Surg Pathol. 1990;14:1156–1164.
Kuo TT, Chan HL. Ossifying dermatofibroma with osteoclast-like giant cells. Am J Dermatopathol. 1994;16:193–195.
Kutchemeshgi M, Barr R, Henderson C. Dermatofibroma with osteoclast-like giant cells. Am J Dermatopathol. 1992;14:397–401.
Le Boit PE, Barr RJ. Smooth-muscle proliferation in dermatofibromas. Am J Dermatopathol. 1994;16:155–160.
Cheng L, Amini SB, Tarif Zaim M. Follicular basal cell hyperplasia overlying dermatofibroma. Am J Surg Pathol. 1997;21:711–718.
Dalziel K, Marks R. Hair follicle-like change over histiocytomas. Am J Dermatopathol. 1986;8:462–466.
Goette DK, Helwig EB. Basal cell carcinomas and basal cell carcinoma-like changes overlying dermatofibromas. Arch Dermatol. 1975;111:589–592.
Morgan MB, Howard HG, Everett MA. Epithelial induction in dermatofibroma: a role for the epidermal growth factor (EGF) receptor. Am J Dermatopathol. 1997;19:35–40.
Herman KL, Kantor GR, Katz SM. Squamous cell carcinoma in-situ overlying dermatofibroma. J Cutan Pathol. 1990;17:385–387.
Zelger BG, Sidoroff A, Zelger B. Combined dermatofibroma: co-existence of two or more variant patterns in a single lesion. Histopathology. 2000;36:529–539.
Schwob VS, Santa Cruz DJ. Palisading cutaneous fibrous histiocytoma. J Cutan Pathol. 1986;13:403–407.
Kuo TT, Hu S, Chan HL. Keloidal dermatofibroma: report of 10 cases of a new variant. Am J Surg Pathol. 1998;22:564–568.
Zelger BG, Calonje E, Zelger B. Myxoid dermatofibroma. Histopathology. 1999;34:357–364.
Soyer HP, Metze D, Kerl H. Granular cell dermatofibroma. Am J Dermatopathol. 1997;19:168–173.
Zelger BG, Steiner H, Kutzner H, Rütten A, Zelger B. Granular cell dermatofibroma. Histopathology. 1998;31:258–262.
Iwata J, Fletcher CD. Lipidized fibrous histiocytoma: clinicopathologic analysis of 22 cases. Am J Dermatopathol. 2000;22:126–134.
Paties C, Vassallo G, Taccogni GL. Clear cell dermatofibroma. Am J Surg Pathol. 1997;21:250–252.
Wambacher-Gasser B, Zelger B, Zelger BG, Steiner H. Clear cell dermatofibroma. Histopathology. 1997;30:64–69.
Aiba S, Terui T, Tagami H. Dermatofibroma with diffuse eosinophilic infiltrate. Am J Dermatopathol. 2000;22:281–284.
Leyva WH, Santa Cruz DJ. Atypical cutaneous fibrous histiocytoma. Am J Dermatopathol. 1986;8:467–471.
Sanchez Yus E, Soria L, de Eusebio E, Requena L. Lichenoid, erosive and ulcerated dermatofibromas. Three additional clinico-pathologic variants. J Cutan Pathol. 2000;27:112–117.
Tamada S, Ackerman AB. Dermatofibroma with monster cells. Am J Dermatopathol. 1987;9:380–387.
Calonje E, Mentzel T, Fletcher CD. Cellular benign fibrous histiocytoma. Clinicopathologic analysis of 74 cases of a distinctive variant of cutaneous fibrous histiocytoma with frequent recurrence. Am J Surg Pathol. 1994;18:668–676.
Calonje E, Fletcher CDM. Aneurysmal benign fibrous histiocytoma. Clinico pathological analysis of 40 cases of a tumour frequently misdiagnosed as a vascular neoplasm. Histopathology. 1995;26:323–332.
Santa Cruz DJ, Kyriakos M. Aneurysmal (“angiomatoid”) fibrous histiocytoma of the skin. Cancer. 1981;47:2053–2061.
Glusac EJ, Barr RJ, Everett MA, Pitha J, Santa Cruz DJ. Epithelioid cell histiocytoma. A report of 10 cases including a new cellular variant. Am J Surg Pathol. 1994;18:583–590.
Singh Gomez C, Calonje E, Fletcher CD. Epithelioid benign fibrous histiocytoma of skin. Clinico-pathological analysis of 20 cases of a poorly known variant. Histopathology. 1994;24:123–129.
Wilson Jones E, Cerio R, Smith N. Epithelioid cell histiocytoma. A new entity. Br J Dermatol. 1989;120:185–195.
Glusac EJ, McNiff JM. Epithelioid cell histiocytoma: a simulant of vascular and melanocytic neoplasms. Am J Dermatopathol. 1999;21:1–7.
Burgdorf WHC, Duray P, Rosai J. Immunohistochemical identification of lysozyme in cutaneous lesions of alleged histiocytic nature. Am J Clin Pathol. 1981;75:162–167.
Gonzalez BS. Benign fibrous histiocytoma of the skin. An immunohistochemical analysis of 30 cases. Pathol Res Pract. 1985;180:486–489.
Abenoza P, Lillemoe T. CD34 and Factor XIIIa in the differential diagnosis of dermatofibroma and dermatofibrosarcoma protuberans. Am J Dermatopathol. 1993;15:429–434.
Prieto VG, Reed JA, Shea CR. Immunohistochemistry of dermatofibromas and benign fibrous histiocytomas. J Cutan Pathol. 1995;22:336–341.
Kutzner H. Expression of the human progenitor cell antigen CD34 (HCPA-1) distinguishes dermatofibrosarcoma protuberans from fibrous histiocytoma in formalin-fixed, paraffin-embedded tissue. J Am Acad Dermatol. 1993;28:613–617.
Kahn HJ, Fekete E, From L. Tenascin differentiates dermatofibroma from dermatofibrosarcoma protuberans: comparison with CD34 and factor XIIIa. Hum Pathol. 2001;32:50–56.
Bruecks AK, Trotter MJ. Expression of desmin and smooth muscle myosin heavy chain in dermatofibromas. Arch Pathol Lab Med. 2002;126:1179–1183.
Zelger BW, Zelger BG, Rappersberger K. Prominent myofibroblastic differentiation: a pitfall in the diagnosis of dermatofibroma. Am J Dermatopathol. 1997;19:138–146.
Guillou L, Gebhard S, Salmeron M, Coindre JM. Metastasizing fibrous histiocytoma of the skin: a clinicopathologic and immunohistochemical analysis of three cases. Mod Pathol. 2000;13:654–660.
Black WC, McGavran MH, Graham P. Nodular subepidermal fibrosis. Arch Surg. 1969;98:296–300. WC, McGavran MH, Graham P. Nodular subepidermal fibrosis. Arch Surg. 1969;98:296–300.