Home > E. Pathology by systems > Reproductive system > Female genital system > Breast > mammary fibroadenoma
mammary fibroadenoma
Tuesday 18 January 2005
breast fibroadenoma, Benign cystosarcoma phyllodes, Cellular fibroadenoma, Cellular adenofibroma, Fetal fibroadenoma, Juvenile adenofibroma; mammary fibroadenoma
| PO | Webpathology | BP |
Definition : The mammary fibroadenoma is a benign mammary biphasic tumor with epithelial and stromal components.
It is a benign breast lesion that result from hyperplasia of the normal lobules.
It is a circumscribed, often large, breast mass usually occuring in adolescent females with stromal and epithelial hypercellularity but lacking the leaf-like growth pattern of phyllodes tumors.
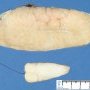
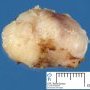
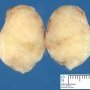
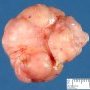
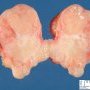
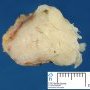
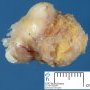
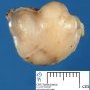
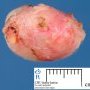
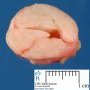
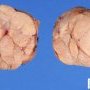
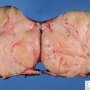
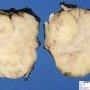
Fibroadenoma is a benign tumor that arises from the epithelium and stroma of terminal duct-lobular unit. Grossly, the fibroadenomas are small, well-demarcated, firm, grayish-pink masses. The cut surface is bulging with a whorled appearance.
The median age at presentation for fibroadenomas is about 25 yrs. and the mean age is about 30 yrs.
Images
Benign phyllodes tumor of the breast, coexisting with fibroadenoma
Digital slides
JRC:5213 : Intracanalicular mammary fibroadenoma.
JRC:5214 : Intracanalicular mammary fibroadenoma.
JRC:5215 : Giant mammary fibroadenoma.
JRC:5217 : Giant mammary fibroadenoma.
JRC:5220 : Mammary fibroadenoma.
Flickr
Types
fibroadenoma, intercanalicular type
fibroadenoma, intracanalicular type
Synopsis
Common
Benign
Usually aged 20–35 years
Multiple in 20%: in same breast or bilaterally
Increase in size during pregnancy
Tend to regress as patient ages
Pathogenesis
Appears to be benign neoplasm of specialized stroma of breast with accompanying epithelial component
Rapidly growing fibroadenomas in immunosuppressed individuals contain Epstein–Barr virus
No differences between fibroadenomas removed from patients taking oral contraceptives and those in controls except occasional formation of acini in former
Macroscopy
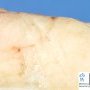
Grossly, the fibroadenomas are small, well-demarcated, firm, grayish-pink masses. The cut surface is bulging with a whorled appearance. The cut surface of fibroadenoma may show slit-like spaces (as seen here). Necrosis is usually absent. The cut surface has multi-lobulated appearance.
sharply demarcated
firm
@<@3 cm diameter
Cut surface: solid, grayish white, bulging
whorl-like pattern
slit-like spaces
No necrosis
Microscopy
Appearance varies and depends on: relative amount and configuration of glandular tissue ans relative amount of connective tissue
- intracanalicular (a misnomer) when connective tissue invaginates into glandular spaces and appears to be within them
- pericanalicular when regular glandular configuration of glands maintained
often both types in same lesion
distinction has no practical connotations
slightly hypercellular stroma but not to a degree that would justify a diagnosis of phylloides tumor.
Tubule cells:
- cuboidal or low columnar
- round uniform nuclei
- rest on myoepithelial cell layer
Stroma:
- usually loose connective tissue rich in acid mucopolysaccharides
- may be partially or totally dense fibrous type
spindle cells:
- predominantly CD34-positive fibroblasts
- admixed with scattered FXIIIa-positive dendrophages
no elastic tissue
consistent with presumed terminal duct–lobular unit (TDLU) origin of lesion
cellularity varies from case to case:
- if unduly hypercellular consider alternative diagnosis phylloides tumor
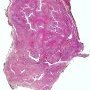
The histologic appearance of fibroadenoma depends upon the relative proportions and the arrangement of glandular and stromal components.
When the stromal connective tissue invaginates into the glandular component, it is labeled intracanalicular pattern. The compressed ducts show linear branching pattern with slit-like lumens (very well seen here). The stromal connective tissue invaginates into the glandular epithelium and appears to be contained within it.
In pericanalicular histologic pattern, the glands maintain their round or oval profiles. There is no prognostic or clinical significance attached to the pericanalicular and intracanalicular patterns. Both may be seen within the same lesion. The tubules and glands in a fibroadenoma are lined by cuboidal or low columnar epithelium with uniform nuclei and surrounded by a myoepithelial layer. The stroma is made up of loose connective tissue. If the stroma is hypercellular, the diagnosis of phyllodes tumor should be excluded.
In some cases, epithelial hyperplasia can be marked. It tends to occur more commonly in juvenile fibroadenomas.
There is a distinct variant of fibroadenoma that is large, hypercellular, and tends to occur in young adolescents, Juvenile Fibroadenoma. They occur more often in African-Americans and may be bilateral. High power view shows proliferation of glandular and stromal elements in a pericanicular growth pattern.There is mild epithelial hyperplasia and stromal hypercellularity. The epithelial hyperplasia is of no significance unless it has sufficient atypia to merit consideration for the diagnosis of carcinoma. Juvenile fibroadenoma have been referred to by a variety of names, as giant fibroadenoma, cellular fibroadenoma etc.
Malignant transformation is seen in 0.1% of cases of fibroadenomas. It usually involves the epithelial component. About 95% of cases are in-situ lesions. In rare cases of malignancy arising in a fibroadenoma, sarcomatous transformation may be seen.
Special Stains and Immunohistochemistry
progesterone receptors: almost universal
estrogen receptors: ≈25% of cases
Predisposition
If multiple and highly myxoid may be component of Carney complex, which includes:
endocrine hyperactivity
cardiac myxoma
cutaneous hyperpigmentation
other abnormalities
other breast abnormalities:
lobular and nodular myxoid changes
ductal adenoma with tubular features
Differential Diagnosis
mammary phylloides tumor
- The mammary fibroadenoma has a slightly hypercellular stroma but not to a degree that would justify a diagnosis of phylloides tumor.
mammary tubular adenoma
mammary adenomyoepithelioma
Genetics
≈20% have clonal chromosome aberrations in stromal component
Prognosis
Low long-term risk for breast carcinoma: increased risk if:
- complex
- ductal hyperplasia
- family history of breast carcinoma
not increased risk if foci of atypical epithelial hyperplasia
Malignant changes in 0.1% of cases:
- usually epithelial component
- most in situ (low-grade intraductal carcinoma)
- +/- entirely within confines of fibroadenoma
- +/- involves surrounding breast
- +/- may represent extension into fibroadenoma by carcinoma
- +/- originating elsewhere in breast
sarcomatous transformation of stroma even rarer
Differential diagnosis: mammary biphasic lesions
adenomyoepithelioma
mammary fibroadenomas
- mammary juvenile fibroadenoma
mammary hamartoma
metaplastic mammary carcinoma
phyllodes tumor
pleomorphic adenoma
gynecomastia
pubertal macromastia
See also
fibroadenomatosis
mammary tumors
Links
http://www.breastpathology.info/fibroadenoma.html
http://www.breastpathology.info/fibro_variants.html
http://www.breastpathology.info/phyllodes.html
References
Wang ZC, Buraimoh A, Iglehart JD, Richardson AL. Genome-wide analysis for loss of heterozygosity in primary and recurrent phyllodes tumor and fibroadenoma of breast using single nucleotide polymorphism arrays. Breast Cancer Res Treat. 2006 Jun;97(3):301-9. PMID: 16791486
Fletcher JA, Pinkus GS, Weidner N, Morton CC. Lineage-restricted clonality in biphasic solid tumors. Am J Pathol. 1991;138:1199–1207.
Kleer CG, Tseng MD, Gutsch DE, Rochford RA, Wu Z, Joynt LK, et al. Detection of Epstein-Barr virus in rapidly growing fibroadenomas of the breast in immunosuppressed hosts. Mod Pathol. 2002;15:759–764.
Fechner RE. Fibroadenomas in patients receiving oral contraceptives. A clinical and pathologic study. Am J Clin Pathol. 1970;53:857–864.
4 - fibroadenomas and spindle cell lesions of the breast. Histopathology. 2001;38:62–67.
Silverman JS, Tamsen A. Mammary fibroadenoma and some phylloides tumour stroma are composed of CD34+ fibroblasts and factor XIIIa+ dendrophages. Histopathology. 1996;29:411–419.
Berean K, Tron VA, Churg A, Clement PB. Mammary fibroadenoma with multinucleated stromal giant cells. Am J Surg Pathol. 1986;10:823–827.
Goodman ZD, Taxy JB. Fibroadenomas of the breast with prominent smooth muscle. Am J Surg Pathol. 1981;5:99–101.
Oberman HA, Nosanchuk HS, Finger JE. Periductal stromal tumors of breast with adipose metaplasia. Arch Surg. 1969;98:384–387.
Shimizu T, Ebihara Y, Serizawa H, Toyoda M, Hirota T. Histopathological study of stromal smooth muscle cells in fibroadenoma of the breast. Pathol Int. 1996;46:442–449.
Arrigoni MG, Dockerty MB, Judd ES. The identification and treatment of mammary hamartoma. Surg Gynecol Obstet. 1971;133:577–582.
Metcalf JS, Ellis B. Choristoma of the breast. Hum Pathol. 1985;16:739–740.
Petrik PK. Mammary hamartoma. Am J Surg Pathol. 1987;11:234–235.
Azzopardi JG. Problems in breast pathology. Bennington JL editors. In: Major problems in pathology. vol. 11:Philadelphia: W.B. Saunders; 1979.
Eusebi V, Azzopardi JG. Lobular endocrine neoplasia in fibroadenoma of the breast. Histopathology. 1980;4:413–428.
Dupont WD, Page DL, Parl FF, Vnencak-Jones CL, Plummer WD, Rados MS, et al. Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med. 1994;331:10–15.
Kuijper A, Mommers EC, van der Wall E, van Diest PJ. Histopathology of fibroadenomas of the breast. Am J Clin Pathol. 2001;115:736–742.
O’Hara MF, Page DL. Adenomas of the breast and ectopic breast under lactational influences. Hum Pathol. 1985;16:707–712.
Dehner LP, Hill DA, Deschryver K. Pathology of the breast in children, adolescents, and young adults. Semin Diagn Pathol. 1999;16:235–247.
Mies C, Rosen PP. Juvenile fibroadenoma with atypical epithelial hyperplasia. Am J Surg Pathol. 1987;11:184–190.
Pike AM, Oberman HA. Juvenile (cellular) adenofibromas. A clinicopathologic study. Am J Surg Pathol. 1985;9:730–736.
Fekete P, Petrek J, Majmudar B, Someren A, Sandberg W. Fibroadenomas with stromal cellularity. A clinicopathologic study of 21 patients. Arch Pathol Lab Med. 1987;111:427–432.
Carstens PHB. Ultrastructure of human fibroadenoma. Arch Pathol. 1974;98:23–32.
Yeh I-T, Francis DJ, Orenstein JM, Silverberg SG. Ultrastructure of cystosarcoma phylloides and fibroadenoma. A comparative study. Am J Clin Pathol. 1985;84:131–136.
Reddick RL, Shin TK, Sawhney D, Siegal GP. Stromal proliferations of the breast. An ultrastructural and immunohistochemical evaluation of cystosarcoma phylloides, juvenile fibroadenoma, and fibroadenoma. Hum Pathol. 1987;18:45–49.
Umekita Y, Yoshida H. Immunohistochemical study of hormone receptor and hormone-regulated protein expression in phylloides tumour: comparison with fibroadenoma. Virchows Arch. 1998;433:311–314.
Carney JA, Toorkey BC. Myxoid fibroadenoma and allied conditions (myxomatosis) of the breast. A heritable disorder with special associations including cardiac and cutaneous myxomas. Am J Surg Pathol. 1991;15:713–721.
Petersson C, Pandis N, Rizou H, Mertens F, Dietrich CU, Adeyinka A, et al. Karyotypic abnormalities in fibroadenomas of the breast. Int J Cancer. 1997;70:282–286.
Carter BA, Page DL, Schuyler P, Parl FF, Simpson JF, Jensen RA, et al. No elevation in long-term breast carcinoma risk for women with fibroadenomas that contain atypical hyperplasia. Cancer. 2001;92:30–36.
Buzanowski-Konakry K, Harrison EG, Payne WS. Lobular carcinoma arising in fibroadenoma of the breast. Cancer. 1975;35:450–456.
Goldman RC, Friedman NB. Carcinoma of the breast arising in fibroadenomas with emphasis on lobular carcinoma. A clinicopathologic study. Cancer. 1969;23:544–550.
McDivitt RW, Stewart FW, Farrow JH. Breast carcinoma arising in solitary fibroadenomas. Surg Gynecol Obstet. 1967;125:572–576.
Diaz NM, Palmer JO, McDivitt RW. Carcinoma arising within fibroadenomas of the breast. A clinicopathologic study of 105 patients. Am J Clin Pathol. 1991;95:614–622.
Fondo EY, Rosen PP, Fracchia AA, Urban JA. The problem of carcinoma developing in a fibroadenoma. Recent experience at Memorial Hospital. Cancer. 1979;43:563–567.
Pick PW, Iossifides IA. Occurrence of breast carcinoma within a fibroadenoma. A review. Arch Pathol Lab Med. 1984;108:590–594.
Curran RC, Dodge OG. Sarcoma of breast, with particular reference to its origin from fibroadenoma. J Clin Pathol. 1962;15:1–16.