Home > E. Pathology by systems > Digestive system > Colon and rectum > colorectal adenoma
colorectal adenoma
Wednesday 12 May 2004
colorectal adenomas ; TA/TVA
Definition: Adenomas, the benign glandular neoplasms that precede colon cancer development, originate from the intestinal epithelium. They occur singly or multiply. When multiple, the patients may have a genetic predisposition syndrome for colorectal cancer.
See also : colorectal adenoma with adenocarcinoma
Images
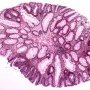
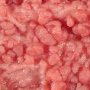
Macroscopy : colorectal tubular adenoma
Endoscopy
Small (@<@ 5 mm) colorectal polyps commonly affect individuals older than 50 years of age and adenomas account for 60% to 66% ofthese small lesions.
The gross endoscopic appearance may suggest the correct diagnosis. However, the endoscopic diagnosis is only correct in 82% ofsmaller polyps, so histologic examination is required for confirmation.
The current wisdomis that distal small adenomas represent biomarkers of risk for colorectal neoplasia, warranting a complete colonoscopy in the patient and lifetime surveillance for colon cancer.
Recently, attention has focused on the development of fluorescence endoscopic imaging and high-resolution chromoendoscopy, which might provide morphologic detail of diminutive colorectal polyps that might correlate with polyp histology and eliminate the need for a biopsy and/or subsequent colonoscopy.
Macroscopy
Grossly, adenomas assume one of three major growth patterns:
(a) pedunculated,
(b) sessile,or
(c) flat or depressed.
Pedunculated and Sessile Adenomas
Most sporadic colorectal adenomas appear as exophytic, mucosal protrusions. They range in size from invisible unicryptal adenomas to large sessile adenomas sometimes measuring > 20 cm in greatest dimension.
Adenoma size gener ally correlates with gross growth pattern. Adenomas measuring only 1 or 2 mm grossly resemble hyperplastic polyps. These minute adenomas have smooth surfaces and lack lobulations, and their color often resembles that of the normal mucosa.
However,when such lesions are examined under a dissecting microscope, they exhibit characteristic pit patterns that differ from those seen in either small carcinomas or hyperplastic polyps.
Polyp architecture depends in part on whether the adenoma has a tubular, villous, or tubulovillous histologic pattern.
The typical tubular adenoma presents as a small, spherical, and variably pedunculated lesion,with its surface broken into lobules by intercommunicating clefts in larger lesions.
Larger lesions appear redder than the surrounding mucosa, unless the patient has melanosis coli, in which case the lesions may appear lighter.
Larger lesions exhibit a lobulated, bosselated or villous, raspberry-like, friable surface.
In surgical material, approximately 90% of adenomas appear variably pedunculated and normal mucosa lines their stalks.
The stalk ranges in length from several millimeters to a few centimeters.
Tubulovillous adenomas tend to be larger than tubular adenomas, with a mean diameter of 19.0 mm.
Villous adenomas fall into three types:
(a) flat, carpet-like masses;
(b) lobulated, bulky, sessile masses;
(c) pedunculated lesions with short, broad pedicles.
Sessile adenomas tend to be large, shaggy lesions covered by fin-gerlike fronds.
Generally, adenomas appear as grossly homogeneous, soft lesions without induration, ulceration, or fixation.
Areas of pigmentation may indicate previous hemorrhage, fibrosis, pseudoinvasion, or previous fulguration.
Areas of ulceration, depression, or firmness suggest the possibility of a coexisting carcinoma.
Villous adenomas can be multiple and often associate with other adenomas, polyps, or separate carcinomas.
Villous adenomas often have ill-defined edges and attach to the mucosa by a broad base, often extending over widemucosal areas.
Because villous adenomas are less well circumscribed than tubular adenomas, with less well-defined edges than pedunculated adenomas, villous adenomas have a greater tendency to recur after local excision.
Large, circumferential, carpeting, benign villous rectal adenomas are rare but problematic. They recur after the initial excision and may require repeated excision or diathermy to control the recurrences.
Adenomas tend to be larger in males and to be largest in the rectum, followed by the ascending colon, cecum, and sigmoid colon.
Adenomas also tend to be larger in individuals with multiple adenomas.
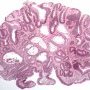
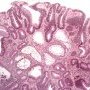
Microscopy
Histologically, adenomas fall into four categories:
tubular adenoma
tubulovillous adenoma
villous adenoma
flat adenoma (or depressed adenoma)
Large or sessile adenomas are generally predominantly villous lesions, whereas smaller, pedunculated adenomas usually display a tubular or tubulovillous architecture.
Although most villous adenomas are large, small villous adenomas do exist.
Some tubular adenomas may become large and sessile, and some pedunculated adenomas exhibit villous features.
Many adenomas histologically show a mixture of both tubular and villous growth patterns.
Types
- colorectal tubular adenoma
The most common adenoma is tubular, accounting for 68% to 87.1% of adenomas in several studies, depending on whether or not one allows for up to a 25% villous component in tubular lesions.
Tubular adenomas maintain their original crypt architecture, but adenomatous epithelium replaces the normal colonic epithelium in liningthe crypts.
Small tubular adenomas always have dysplastic (adenomatous) surface epithelium overlying non-dysplastic epithelium in the crypt base.
Lamina propria separates the closely packed adenomatous crypts. The lamina propria may contain increased number of lymphocytes, plasma cells, and eosinophils.
When the adenomatous tubules grow, they may branch, sometimes producing an irregular architecture.
In pedunculated polyps, adenomatous epithelium remains confined to the mucosa ofthe head ofthe polyp.
The stalk consists of normal mucosa, including the muscularis mucosae and submucosal tissue, incontinuity with the major part of the bowel wall.
Focal cystic tubular dilation, inflammation, hemorrhage, or erosion can all secondarily affect adenomas, especially at their surface.
Small superficial tubular adenomas composed of an adenomatous epithelium that produces some mucin are occasionally overlooked.
Their neoplastic nature can be confirmed using an antibody to Ki-67. The strongly immunoreactive cells will be confined to the surface,and more weakly positive cells will be at the crypt bases.
Colorectal Villous Adenomas
Approximately 20% of a symptomatic persons screened by colonoscopy have villous adenomas, a third of which contain high-grade dysplasia.
Slightly @<@2% contain invasive carcinoma.
Villous adenomas consist of elongated finger-like non-branching fronds of dysplastic epithelium extending outward from the muscularis mucosae to the colonic lumen.
The villi contain cores oflamina propria covered by a single layer of adenomatous epithelium
It has been defined that villous lesions as those that contain
> 80% of a villous component.
Colorectal Tubulovillous Adenomas
Tubulovillous adenomas contain a mixture of both tubular and villous growth patterns or have broad villi containing tubular structures. The villi may be blunt andshort.
Konishi and Morson define tubulovillous lesions as those that contain from 20% to 79% villous components.
Fung and Goldman estimated that a villous component is present in 35% to 75% of all adenomas measuring >1 cm in greatest diameter.
Types
unicrypric adenoma
adenoma containing carcinoma
flat adenoma (depressed adenoma)
Vienna classification applied to colorectal adenomas
Colorectal adenomas can be histologically classified according to the categories listed in Vienna:
category 3, low-grade dysplasia;
4.1, high-grade dysplasia;
4.2, carcinoma in situ;
4.3, suspicious of intramucosal carcinoma;
5.1, intramucosal carcinoma;
5.2, submucosal carcinoma.
Adenomas can be associated with dysplasia (categories 3 and 4.1) or with carcinoma (categories 4.2, 4.3, 5.1 and 5.2).
On basis of their configuration, adenomas are classified into tubular, tubulovillous, villous, serrated, microtubular and combined phenotypes (i.e. other than tubulovillous).
Cell Types in Adenomas
Adenomas contain a mixture of variably differentiated absorptive cells, goblet cells, intermediate cells, endocrine cells, and Paneth cells.
An abrupt transition between adenomatous andnormal colonic epithelial cells is often seen, with the adenomatous cells displacing the non-neoplastic cells of the crypt creatinga “snowplow” effect.
Most adenomas show some ability to partially differentiate into immature mucus-producing cells called oligomucous cells.
However, mucus content ofthe adenomatous epithelium varies.
Adenomas may demonstrate true goblet cell formation,although these cells often have an eccentric nucleus and are referred to as dystrophic goblet cells.
Occasionally, large numbers of mucin-producing cells are present in villous adenomas, especially those associated with potassium loss.
Endocrine cells are discernible in 59% to 85% of adenomas if special stains are used to detect them.
Paneth cellsare present in approximately 10%, and squamous differentiation occurs in approximately 4% of lesions.
Paneth cells are easily recognized in hematoxylin and eosin–stained sections due to their prominent supranuclear, eosinophilic, cytoplasmic granules.
The Paneth cells are neoplastic, as evidenced by their cytologic features.
Some cells exhibit both mucinous and Paneth cell differentiation.
Adenomas that contain squamous epithelium probably act as the precursor lesions for adenosquamous carcinomas, adenoacanthomas, or pure squamous cell carcinomas.
Adenomas may also contain foci of osseous metaplasia, melanocytes, or areas of gastric mucosa.
The presence of these various cell types reflects stem cell potential to differentiate along several cell lineages.
The presence ofthese various cell types in adenomas has no clinical significance.
Muscle Fibers in Adenomas
When adenomas form, the underlying muscularis mucosae frays, sending small finger like muscular extensions short distances into the overlying interglandular stroma.
The muscular component is most evident at thejunction of the head and stalk ofthe adenoma, where it may form a broad muscular zone.
The muscularis mucosae ofthe stalk merges with the muscular zone ofthe adenoma.
The deeper border ofthe muscular zone is not as distinct in pedunculated lesions as in sessile ones.
The thick muscular zone disappears when invasive cancer develops in the adenoma.
Vasculature in Adenomas
The lymphatic plexus begins in the area ofthe muscularis mucosae, and it may accompany the distorted fibers of the muscularis into the overlying mucosa.
However, lymphatics never extend higher than the bases of the crypts.
The microvasculature of adenomas has an organization similar to that of the normal colon, but the capillaries and venules appear elongated and have increased diameters compared to the vessels present in the normal lamina propria.
Microvessel density increases in the spaces between the neoplastic cells. It also increases as the severity of the dysplasia increases.
Differential diagnosis
adenoma with pseudocarcinomatous entrapment (Pseudoinvasion)
Cytogenetics
gain of chromosome 7 (most frequent anomaly)
trisomy 20
tumoral trisomy 13
nosomy 18
arrangement of chromosome 1
- 1p deletion
- del(1)(p36) (1p36 deletion)
loss of 8p
gain of 8q
Molecular biology
Genetic alterations occur during the adenoma-carcinoma sequence of colon cancer formation and drive the initiation and progression of colon cancer formation.
The aberrant methylation of genes is an alternate, epigenetic mechanism for silencing tumor suppressor genes in colon cancer.
Open references
Molecular validation of the modified Vienna classification of colorectal tumors.
Sugai T, Habano W, Uesugi N, Jiao YF, Nakamura S, Sato K, Chiba T, Ishii M.
J Mol Diagn. 2002 Nov;4(4):191-200.
PMID: 12411586 Free
The Vienna classification of gastrointestinal epithelial neoplasia.
Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, Hattori T, Hirota T, Itabashi M, Iwafuchi M, Iwashita A, Kim YI, Kirchner T, Klimpfinger M, Koike M, Lauwers GY, Lewin KJ, Oberhuber G, Offner F, Price AB, Rubio CA, Shimizu M, Shimoda T, Sipponen P, Solcia E, Stolte M, Watanabe H, Yamabe H.
Gut. 2000 Aug;47(2):251-5.
PMID: 10896917 Free
References
The Vienna classification applied to colorectal adenomas.
Rubio CA, Nesi G, Messerini L, Zampi GC, Mandai K, Itabashi M, Takubo K.
J Gastroenterol Hepatol. 2006 Nov;21(11):1697-703.
PMID: 16984592
Kim YH, Petko Z, Dzieciatkowski S, Lin L, Ghiassi M, Stain S, Chapman WC, Washington MK, Willis J, Markowitz SD, Grady WM. CpG island methylation of genes accumulates during the adenoma progression step of the multistep pathogenesis of colorectal cancer. Genes Chromosomes Cancer. 2006 Aug;45(8):781-9. PMID: 16708352
Bomme L, Bardi G, Pandis N, Fenger C, Kronborg O, Heim S. Chromosome abnormalities in colorectal adenomas: two cytogenetic subgroups characterized by deletion of 1p and numerical aberrations. Hum Pathol. 1996 Nov;27(11):1192-7. PMID: 8912830