Home > E. Pathology by systems > Skin > fibrous hamartoma of infancy
fibrous hamartoma of infancy
Friday 10 March 2006
juvenile fibrous hamartoma, infantile fibrous hamartoma
Definition: Fibrous hamartoma of infancy is an uncommon fibroproliferative lesion of the subcutaneous tissue that is present at birth or develops in the first 2 years of life.
Digital slides
HPC:21 (HPC:21)
HPC:65 (HPC:65)
HPC:152 (HPC:152) : Fibrous hamartoma of infancy
HPC:137 (HPC:137)
HPC:65 (HPC:65)
JRC:106 : Fibrous hamartoma of infancy
Images
Fibrous hamartoma of infancy
- https://twitter.com/mariaramir/status/722563420537688064
- https://twitter.com/ARP_Press/status/873953789606277122
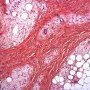
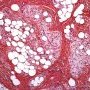
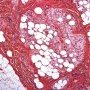
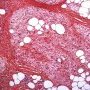
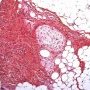
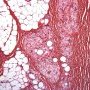
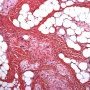
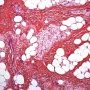
Pediatric, benign, poorly circumscribed, superficial soft tissue mass with three components: dense fibrocollagenous tissue, loosely textured areas of immature rounded mesenchymal cells and mature fat. It occurs most frequently in the anterior or posterior axillary fold, followed by the upper arm and shoulder, thigh, groin, back, and forearm. Rarely arises in the hands and feet.
Localization
Fibrous hamartoma of infancy most commonly occurs around the shoulder, axilla, and upper arms, but cases involving the scalp, inguinal region, scrotum, perianal area, and lower extremities have been reported.
These tumors are usually solitary and have previously been thought to occur only rarely in the hands and feet.
Epidemiology
Accounting for 0.02% of all benign soft tissue, although is one of the relatively more common tumors of fibrous tissue in early childhood.
Fibrous hamartoma of infancy occurs in the first 2 years of life and may even be present at birth. There is a male predominance of 3 : 1 (M>F). They do not occur after puberty, and there is boy predominance.
Usually @<@ 5 years of age,
- majority @<@ 2 years of age
25% congenital
Clinical synopsis
Benign soft tissue mass of childhood. The clinical course is benign, despite its infiltrative appearance and tendency to local recurrence.
Fibrous hamartoma of infancy is almost always a solitary lesion, rapidly growing, freely movable mass in the subcutis or dermis, occasionally attached to underlying fascia and rarely involving skeletal muscle.
It has been reported in an infant with Williams’ syndrome.
Macroscopy
The subcutaneous tissue of excised lesions has a glistening gray-white appearance interspersed with fatty tissue. The involved area measures 2–8 cm in maximum diameter.
Usually poorly circumscribed, grey-white tissue alternating with yellow fat. Most lesions are less than 5 cm in diameter, tumors rarely reach larger than 10 cm.
Microscopy
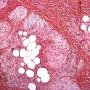
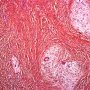
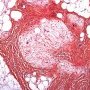
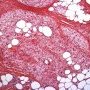
FHI is characterized by three distinct components forming organoid structures. The well defined intersecting trabeculae of dense fibrocollagenous tissue are composed of fibroblastic and myofibroblastic spindle cells with bland, straight or wavy nuclei separated by varying amounts of collagen.
Between fibrous trabeculae are islands of immature-appearing , small, rounded or stellate, primitive mesenchymal cells with scant cytoplasm embedded in myxoid matrix containing abundant hyaluronidase-sensitive acid mucopolysaccharides. The primitive myxoid areas are frequently oriented around small veins.
The mature fat component is interspersed among the other two components. The relative proportions of these components vary between cases.
Fibrous hamartoma of infancy has poorly defined margins. Fibrous hamartoma of infancy is centered on the subcutis.
There are three different tissue components:
interlacing trabeculae of fibrocollagenous tissue
small nests of loosely arranged mesenchymal cells
interspersed mature fat.
Microscopic features include an admixture of mature adipose cells, nodular aggregates of immature mesenchymal elements with interlacing bands of fibrocollagenous tissue, and spindled fibroblastic cells.
The fibrous trabeculae vary in thickness and arrangement and contain spindle-shaped cells; they express vimentin but not S100 protein.
Desmin and smooth muscle actin are found in the fascicular-fibroblastic regions.
The myxoid areas are more cellular, with immature oval or stellate cells, sometimes having a whorled pattern. Sparse lymphocytes may be present in the stroma.
An organoid growth pattern justified the term hamartoma.
The overlying skin frequently shows eccrine changes that include hyperplasia, duct dilatation, intraluminal papillary formations, and squamous syringometaplasia.
Primitive mesenchymal cells may replace the normal eccrine gland stroma. Increased numbers of terminal hair follicles, and epidermal basaloid follicular hyperplasia have also been reported.
Synopsis
Trunk and limbs
- usually upper arm and shoulder
Painless solitary subcutaneous mass
May recur locally
Local excision treatment of choice
Organoid distribution of three components
- fat
- fibrous tissue
- immature mesenchyme
Highly variable amounts of each component
In older children, lesions may show extensive sclerosis
Fatty lobules with fibrocollagenous spindle cell septae
Islands of immature stellate cells in variably myxoid matrix
Mature fat
Overlying skin may show changes - especially eccrine changes
- hyperplasia, duct dilatation, intraluminal papillary formations
possible separate lesions
+/- infiltration into the superficial muscle
+/- infiltration and entrapment of nerves
possible rapid recurrence after initial surgery
organoid pattern with components of fat, fibrous tissue and immature mesenchyme.
Localization
spinal FHI (16933384)
knee (16532345), wrist (11436895)
Variants
multiple nodules with hypertrichosis (16700836)
recurrent FHI (15645249)
Immunochemistry
Spindle cells vimentin+
Scattered CD34+
Desmin−
Electron microscopy
The constituent cells have the features of myofibroblasts, although some fibroblasts are also present. Fibroblasts alone were present in one reported case. Primitive mesenchymal cells are present in the immature-appearing areas.
fibroblasts
-/+ myofibroblasts
/+ fusiform banded fibers (Luse bodies)
Molecular biology
EGFR Exon 20 Insertion/Duplication Mutations Characterize Fibrous Hamartoma of Infancy. (27631514)
Cytogenetics
t(2;3)(q31;q21) (15794678)
complex translocation (6;12;8)(q25;q24.3;q13) (17116490)
complex structural rearrangements involving chromosomes 1, 2, 4, and 17. (20633773)
Differential diagnosis
The diagnosis of fibrous hamartoma of infancy is based on the simultaneous presence of three tissue types, well-defined bundles, or trabeculae of dense fibrocollagenous tissue; loosely textured cellular areas of immature fibroblasts, mesenchymal elements in a myxoid matrix; and mature adipose cells interposed between the other two elements.
- Lipofibromatosis is said to have a predilection for hands and feet, unlike infantile fibrous hamartoma, which more commonly occurs in the proximal upper and lower limb girdles.
- Additionally, while both types of lesions have mature adipocytes, the characterizing feature of infantile fibrous hamartoma is primitive organoid nests of mesenchymal cells in a loose myxoid matrix.
- Lesions of lipofibromatosis that have had long-term follow-up and although incompletely excised have not progressed but equally have not necessarily regressed. Local recurrence is common, and complete removal is preferable.
Association
Williams syndrome (17326745)
Prognosis and management
Fibrous hamartoma of infancy is benign and usually cured by local excision. Rare recurrences are cured by reexcision.
Fibrous hamartoma of infancy does not spontaneously regress and may recur following surgical resection.
This tumor should be treated by complete excision; an aggressive approach should be avoided, as the overall prognosis is excellent.
See also
Tumors
- fibrous tumors
Fibrous hamartoma of infancy is an uncommon fibroproliferative lesion of uncertain histogenesis that occurs only in infancy and childhood.
Paywall References
EGFR Exon 20 Insertion/Duplication Mutations Characterize Fibrous Hamartoma of Infancy. Park JY, Cohen C, Lopez D, Ramos E, Wagenfuehr J, Rakheja D.
Am J Surg Pathol. 2016 Dec;40(12):1713-1718. PMID: 27631514
Cytogenetic characterization of a fibrous hamartoma of infancy with complex translocations. Tassano E, Nozza P, Tavella E, Garaventa A, Panarello C, Morerio C. Cancer Genet Cytogenet. 2010 Aug;201(1):66-9. PMID: 20633773
Rougemont AL, Fetni R, Murthy S, Fournet JC. A complex translocation (6;12;8)(q25;q24.3;q13) in a fibrous hamartoma of infancy. Cancer Genet Cytogenet. 2006 Dec;171(2):115-8. PMID: 17116490
Scott DM, Pena JR, Omura EF. Fibrous hamartoma of infancy. J Am Acad Dermatol. 1999 Nov;41(5 Pt 2):857-9. PMID: 10534670
Dickey GE, Sotelo-Avila C. Fibrous hamartoma of infancy: current review. Pediatr Dev Pathol. 1999 May-Jun;2(3):236-43. PMID: 10191347
Groisman G, Lichtig C. Fibrous hamartoma of infancy: an immunohistochemical and ultrastructural study. Hum Pathol. 1991 Sep;22(9):914-8. PMID: 1916752
Maung R, Lindsay R, Trevenen C, Hwang WS. Fibrous hamartoma of infancy. Hum Pathol. 1987 Jun;18(6):652-3. PMID: 3596584
Greco MA, Schinella RA, Vuletin JC. Fibrous hamartoma of infancy: an ultrastructural study. Hum Pathol. 1984 Aug;15(8):717-23. PMID: 6540239
Mitchell ML, di Sant’Agnese PA, Gerber JE. Fibrous hamartoma of infancy. Hum Pathol. 1982 Jun;13(6):586-8. PMID: 7076241