Home > D. General pathology > malignant hyperthermia
malignant hyperthermia
Tuesday 6 September 2005
Definition: Malignant hyperthermia (MH) is a pharmacogenetic disorder of skeletal muscle that presents as a hypermetabolic response to potent volatile anesthetic gases such as halothane, sevoflurane, desflurane and the depolarizing muscle relaxant succinylcholine, and rarely, in humans, to stresses such as vigorous exercise and heat.
The incidence of MH reactions ranges from 1:5,000 to 1:50,000-100,000 anesthesias. However, the prevalence of the genetic abnormalities may be as great as one in 3,000 individuals.
Malignant hyperthermia susceptibility was first recognized by Denborough et al. in 1962. This is a well-known but uncommon condition, characterized by the development of a hypermetabolic reaction (tachypnea, tachycardia, rigidity, acidosis, rhabdomyolysis, and hyperthermia) when volatile anesthetics and depolarizing neuromuscular blockers are administered together to a susceptible individual.
Malignant hyperthermia susceptibility is an autosomal-dominant condition with multifactorial inheritance. The condition is subdivided into malignant hyperthermia susceptibility 1 to 6.
MH affects humans, certain pig breeds, dogs, horses, and probably other animals. In humans the syndrome is inherited in autosomal dominant pattern, while in pigs in autosomal recessive.
The classic signs of MH include hyperthermia to marked degree, tachycardia, tachypnea, increased carbon dioxide production, increased oxygen consumption, acidosis, muscle rigidity, and rhabdomyolysis, all related to a hypermetabolic response. The syndrome is likely to be fatal if untreated.
Diagnosis
Early recognition of the signs of MH, specifically elevation of end-expired carbon dioxide, provides the clinical diagnostic clues.
Recognition of a family history of this disorder, or of previous clinical signs of malignant hyperthermia on exposure to an anaesthetic agent in an individual, is essential.
Prevention of the hypermetabolic reaction with dantrolene (an inhibitor of calcium release from the sarcoplasmic reticulum) is the mainstay of management.
When the reaction occurs, the use of anesthetics must be discontinued immediately, and active support measures must be undertaken (e.g., core cooling or respiratory support) concurrent with an administration of dantrolene sodium.
Pathophysiology
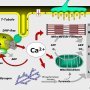
The pathophysiologic changes of MH are due to uncontrolled rise of myoplasmic calcium, which activates biochemical processes related to muscle activation.
Due to ATP depletion, the muscle membrane integrity is compromised leading to hyperkalemia and rhabdomyolysis. In most cases, the syndrome is caused by a defect in the ryanodine receptor.
Etiology
Over 90 mutations have been identified in the RYR-1 gene located on chromosome 19q13.1, and at least 25 are causal for MH. Diagnostic testing relies on assessing the in vitro contracture response of biopsied muscle to halothane, caffeine, and other drugs.
Prognosis
The mortality from MH has dropped from over 80% thirty years ago to less than 5%.
Etiology
Locus MHS1 at 19q13.1: mutation in the RYR1 gene coding for ryanodine receptor (MIM.180901)
Locus MHS2 at 17q11.2-q24
Locus MHS3 at 7q21-q22
Locus MHS4 at 3q13.1
Locus MHS5 at 1q32:
Locus MHS6 at 5p: mutation in the CACNA1S gene
In some families with susceptibility to malignant hyperthermia, additional susceptibility loci on chromosome 3 were documented, as was the role of voltage-dependent calcium channels.
RYR1-associated malignant hyperthermia
RYR1 gene codes for ryanodine receptor (MIM.180901).
Mutations of the RYR1 gene on chromosome 19q, encoding for ryanodine receptor type 1, account for the majority of cases. This receptor is a skeletal muscle calcium channel located in the sarcoplasmic reticulum, responsible for the release of calcium in the sarcoplasmic reticulum, allowing muscle contraction.
Mutation of this channel then leads to an abnormal sustained increase in myoplasmic calcium concentration in skeletal muscle, with the resulting potential for developing malignant hyperthermia.
References
Rosenberg H, Davis M, James D, Pollock N, Stowell K. Malignant hyperthermia. Orphanet J Rare Dis. 2007 Apr 24;2:21. PMID: 17456235
Nelson TE. Malignant hyperthermia: a pharmacogenetic disease of Ca++ regulating proteins. Curr Mol Med. 2002 Jun;2(4):347-69. PMID: 12108947