Home > E. Pathology by systems > Endocrine system > Thyroid > C-cell hyperplasia
C-cell hyperplasia
Monday 3 November 2003
Digital cases
![]() Case 141 (HPC:141) : Thyroid C cell hyperplasia in MEN2a
Case 141 (HPC:141) : Thyroid C cell hyperplasia in MEN2a
Definition: Hyperplasia of C-cells is defined by the presence of at least 50 thyroid C-cells per one low power field (100X). (8616768)
CCH is a potential preneoplastic condition even in the absence of germ-line mutations in the RET protooncogene and seems to be more common in males.
Synopsis
![]() Precursor of medullary carcinoma of thyroid in familial / inherited forms of medullary carcinoma of thyroid
Precursor of medullary carcinoma of thyroid in familial / inherited forms of medullary carcinoma of thyroid![]() Groups of interfollicular C-cells
Groups of interfollicular C-cells
- focal
- nodular
- diffuse
![]() Multiple clusters of more than 6 C-cells or >50 C-cells / low power field
Multiple clusters of more than 6 C-cells or >50 C-cells / low power field
- calcitonin
- chromogranin+
- Difficult distinction in some cases from microinvasive thyroid medullary carcinoma
Microscopy
Although hyperplasia of C-cells has been described in association with various pathologic and physiologic conditions, criteria for its diagnosis are poorly defined.
Neoplastic C-cell hyperplasias, as proposed by Perry et al. in 1996, is defined as the presence of intrafollicular C-cells with nuclear pleomorphism morphologically distinct from the follicular cells and, therefore, recognizable on hematoxylin and eosin-stained sections.
On calcitonin-stained slides, slides can be screened for the presence of C cells. Slides with the highest estimated C-cell density of each lobe are counted for the number of C cells present/visual field at a 100× magnification (low-power field [LPF]).
According to the predominant growth pattern of C-cells, C-cell hyperplasias are classified morphologically into focal, diffuse, nodular, or neoplastic CCH.
- Focal CCH is defined by a segmental proliferation pattern of C cells within thyroid follicles.
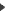 diffuse C-cell hyperplasia
diffuse C-cell hyperplasia - Diffuse CCH is diagnosed when C-cells formed an intrafollicular, circumferential collar around the more centrally located follicular epithelial cells.
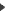 nodular C-cell hyperplasia
nodular C-cell hyperplasia - Nodular CCH was diagnosed when there is complete obliteration of the follicular lumen by hyperplastic C cells.
Areas of C-cell proliferation suspected of early infiltration are regarded as carcinomas if a focal loss or reduplication of basement membrane was observed through immunohistochemistry.
Nota bene: Both neoplastic and physiologic C-cell proliferations have been lumped together under the umbrella designation of C-cell hyperplasia (CCH), creating considerable confusion among clinicians and pathologists.
Physiologic or reactive CCH is not recognized with certainty on H&E stains due to morphologic similarities between C-cells and adjacent follicular cells. Detection of this form of hyperplasia, which was predominantly diffuse, required calcitonin immunostains and quantitative analysis.
Conversely, nodular C-cell hyperplasia and diffuse neoplastic C-cell hyperplasia is easily identified with conventional H&E stains at the periphery of 92% familial medullary thyroid carcinomas (FMTC).
Physiologic CCH vs neoplastic CCH
Physiologic CCH and neoplastic CCH are biologically and morphologically distinct entities. Physiologic CCH cannot be recognized with certainty with conventional stains and requires immunohistochemistry and quantitative analysis for diagnosis.
Neoplastic CCH consists of mildly to moderately atypical C-cells that can be identified with H & E stained sections. Consequently, the number of C-cells is of no importance for the diagnosis of neoplastic CCH which is considered to be the precursor (medullary carcinoma in situ) of invasive medullary carcinoma.
The C-cells in neoplastic CCH are large, mildly to moderately atypical, and confined within the basement membrane of thyroid follicles. Moreover, these cells are cytologically indistinguishable from those of invasive MTC cells.
In some cases, neoplastic C-cell hyperplasia is the only pathologic finding on thyroidectomy performed for elevated serum calcitonin levels detected via provocative biochemical screening or identification of the mutated RET proto-oncogene by genetic analysis.
CCH accompanying MTC
Surgical pathologists have to be cautious when interpreting CCH accompanying MTC as an indicator of familial risk. Calcitonin screening for all patients seen for various thyroid and parathyroid disorders will detect sporadic MTC in early stages and, hence, offers the possibility to improve the prognosis of sporadic MTC.
Etiological types
![]() familial C-cell hyperplasia and/or neoplasia (neoplastic CCH)
familial C-cell hyperplasia and/or neoplasia (neoplastic CCH) ![]() reactive C-cell hyperplasia
reactive C-cell hyperplasia![]() physiologic C-cell hyperplasia
physiologic C-cell hyperplasia
Etiology
![]() RET-associated C-cell hyperplasia
RET-associated C-cell hyperplasia
![]() PTEN-associated tumor syndromes (15067177)
PTEN-associated tumor syndromes (15067177)
- Cowden disease (CD)
- Bannayan-Ruvalcaba-Riley syndrome (BRRS)
Neoplastic CCH
Neoplastic CCH may be the precursor of familial MTC and sporadic MTC.
Of patients who fulfilled the criteria of neoplastic CCH, only some have a mutation in the RET protooncogene.
CCHs and evolving microscopic MTC had nearly identical morphologic features in some patients with multiple endocrine neoplasia MEN2A compared to patients with a sporadic microcarcinoma that evolved from neoplastic CCH.
Differential diagnosis
![]() intraglandular spread of thyroid medullary carcinoma
intraglandular spread of thyroid medullary carcinoma![]() solid cell nests
solid cell nests![]() palapation thyroiditis
palapation thyroiditis![]() microinvasive medullary carcinoma
microinvasive medullary carcinoma
See also
![]() medullary thyroid carcinoma (MTC)
medullary thyroid carcinoma (MTC)![]() abnormal distribution of C-cells
abnormal distribution of C-cells
References
![]() Abnormal distribution and hyperplasia of thyroid C-cells in PTEN-associated tumor syndromes. Zambrano E, Holm I, Glickman J, Huang S, Perez-Atayde A, Kozakewich HP, Shamberger RC, Nose V. Endocr Pathol. 2004 Spring;15(1):55-64. PMID: 15067177
Abnormal distribution and hyperplasia of thyroid C-cells in PTEN-associated tumor syndromes. Zambrano E, Holm I, Glickman J, Huang S, Perez-Atayde A, Kozakewich HP, Shamberger RC, Nose V. Endocr Pathol. 2004 Spring;15(1):55-64. PMID: 15067177
![]() Kaserer K, Scheuba C, Neuhold N, Weinhausel A, Vierhapper H, Haas OA, Niederle B. C-cell hyperplasia and medullary thyroid carcinoma in patients routinely screened for serum calcitonin. Am J Surg Pathol. 1998 Jun;22(6):722-8. PMID: 9630179
Kaserer K, Scheuba C, Neuhold N, Weinhausel A, Vierhapper H, Haas OA, Niederle B. C-cell hyperplasia and medullary thyroid carcinoma in patients routinely screened for serum calcitonin. Am J Surg Pathol. 1998 Jun;22(6):722-8. PMID: 9630179
![]() DeLellis RA. Multiple endocrine neoplasia syndromes revisited. Clinical, morphologic, and molecular features. Lab Invest. 1995 May;72(5):494-505. PMID: 7745945
DeLellis RA. Multiple endocrine neoplasia syndromes revisited. Clinical, morphologic, and molecular features. Lab Invest. 1995 May;72(5):494-505. PMID: 7745945
![]() McDermott MB, Swanson PE, Wick MR. Immunostains for collagen type IV discriminate between C-cell hyperplasia and microscopic medullary carcinoma in multiple endocrine neoplasia, type 2a. Hum Pathol. 1995 Dec;26(12):1308-12. PMID: 8522302
McDermott MB, Swanson PE, Wick MR. Immunostains for collagen type IV discriminate between C-cell hyperplasia and microscopic medullary carcinoma in multiple endocrine neoplasia, type 2a. Hum Pathol. 1995 Dec;26(12):1308-12. PMID: 8522302
![]() Biddinger PW, Brennan MF, Rosen PP. Symptomatic C-cell hyperplasia associated with chronic lymphocytic thyroiditis. Am J Surg Pathol. 1991 Jun;15(6):599-604. PMID: 1674409
Biddinger PW, Brennan MF, Rosen PP. Symptomatic C-cell hyperplasia associated with chronic lymphocytic thyroiditis. Am J Surg Pathol. 1991 Jun;15(6):599-604. PMID: 1674409
![]() Wolfe HJ, Delellis RA. Familial medullary thyroid carcinoma and C cell hyperplasia. Clin Endocrinol Metab. 1981 Jul;10(2):351-65. PMID: 7285384
Wolfe HJ, Delellis RA. Familial medullary thyroid carcinoma and C cell hyperplasia. Clin Endocrinol Metab. 1981 Jul;10(2):351-65. PMID: 7285384
![]() Perry A, Molberg K, Albores Saavedra J. Physiologic versus neoplastic C-cell hyperplasia of the thyroid: separation of distinct histologic and biologic entities. Cancer 1996;77:750-6. (8616768)
Perry A, Molberg K, Albores Saavedra J. Physiologic versus neoplastic C-cell hyperplasia of the thyroid: separation of distinct histologic and biologic entities. Cancer 1996;77:750-6. (8616768)
![]() McDermott MB, Swanson PE, Wick MR. Immunostains for collagen type IV discriminate between C-cell hyperplasia and microscopic medullary carcinoma in multiple endocrine neoplasia, type 2a. Hum Pathol 1995;26:1308-312. PMID: 8522302
McDermott MB, Swanson PE, Wick MR. Immunostains for collagen type IV discriminate between C-cell hyperplasia and microscopic medullary carcinoma in multiple endocrine neoplasia, type 2a. Hum Pathol 1995;26:1308-312. PMID: 8522302